3696
Reproducibility of non-localized 13C-MRS using a quadrature surface coil for assessing hepatic carbohydrate metabolism in humans1Institute for Clinical Diabetology, German Diabetes Center, Leibniz Institute for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, 2German Center for Diabetes Research (DZD e.V.), München-Neuherberg, Germany, 3Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 4Department of Endocrinology and Diabetology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany
Synopsis
Keywords: Spectroscopy, Spectroscopy, 13C-MRS, Quadrature Coil, Absolute Quantification
We determined day-to-day variation and intra-session reproducibility of 13C-MRS based hepatic glycogen quantification by phantom replacement, using a custom-created rigid quadrature surface coil and determining the C1-glycogen signal in eight healthy volunteers. The quadrature coil allows measurements at high distances, which can be important for applications in overweight and obese people. Coefficients of variation were less than 22% for day-to-day variation and less than 9% for intra-session reproducibility (with repositioning of volunteer). In conclusion, our 13C-MRS protocol yields robust absolute concentrations of the hepatic glycogen and the day-to-day variation assessed here can be the basis for sample size calculations.
Introduction
Non-invasive quantification of carbon metabolites in the liver is an established and valuable tool to investigate glycogen metabolism in vivo. Glycogen metabolism is essential for glucose homeostasis and it was shown that individuals with type 2 diabetes mellitus (T2DM) are characterized by lower hepatic glycogen content and an impaired glycogen metabolism, compared to healthy humans1,2. However, limited sensitivity of standard single loop coils in combination with the fact that most individuals with T2DM are overweight or obese, makes the investigation of hepatic glycogen in these people challenging and signal to noise ratio (SNR) is generally very low. To address the high distance of the region of interest (liver) from the coil, a custom-created quadrature coil was designed to perform 13C-MRS in the liver with a non-flexible design (rigid housing) to facilitate phantom-replacement measurements with identical coil configuration. Furthermore, adiabatic pulses can be used to increase SNR. While the application of these measurements is well described in literature, reports on the reproducibility of the measurements are limited to protocols employing block pulses and rather small single loop 13C-coils, which is not optimal in volunteers with a high BMI. The aim of this study is to estimate the reproducibility of 13C-MRS measurements to assess absolute concentration of hepatic glycogen content with a protocol that supports measurements in volunteers with overweight or obesity. Determining the reproducibility of the measurement within a session and in between different days is important in order to estimate potential effect sizes and to perform sample size calculation.Methods
Eight healthy volunteers (7 male/1 female; mean ± standard deviation (SD) 37.1 ± 11.0 years; body mass index (BMI) 27.3 ± 4.2 kg/m2) participated in the study.All volunteers underwent measurements on three different days within a week in order to assess the day-to-day variation. Additionally, five of the eight volunteers were measured a fourth time after three months. In order to achieve standardized physiological conditions, each volunteer was measured at the same time of the day after a 4.5 hours fasting. To assess intra-session variation, three volunteers were examined three times in a single session to estimate reproducibility without coil repositioning followed by two more measurements with repositioning of the volunteer.
Measurements were performed on a clinical 3-T MR system (Philips Achieva dStream, Best, the Netherlands) using a custom-created dual-tuned curved 1H/13C quadrature surface coil (transmit-receive coil; RAPID Biomedical, Rimpar, Germany) with total 1H/13C loop sizes of 250x190 mm and 220x160 mm in quadrature arrangement, respectively.
Liver spectra were obtained during free breathing using a pulse-acquire sequence with a 9.53 ms hyperbolic secant (HS) adiabatic pulse for excitation (excitation bandwidth 655 Hz), repetition time (TR) 435 ms, number of signal averages (NSA) 3000, total acquisition time 22 min, samples (N) 256, spectral bandwidth (BW) 8 kHz, narrowband decoupling (100% of acquisition, 84 Hz downfield of water, B1 amplitude 7 μT) and shim voxel size 60x60x60 mm3.
Spectra of the external reference, containing 530 μl of 1-13C formic acid (FA) in a glass sphere fixed in the middle of the coil housing, were acquired in separate scans using a pulse-acquire sequence with an excitation block pulse (duration 0.43 ms, excitation bandwidth 2.33 kHz), TR 8 s, NSA 16, total acquisition time 2 min, N 256, BW 6 kHz, without decoupling and shim voxel size 80x80x80 mm3.
Absolute quantification of C1-glycogen in millimolar (mM) concentration was achieved using the phantom-replacement method with an in-house mixed phantom containing 250 mM glycogen (cylindrical phantom, volume 2 l, diameter 16 cm, height 19 cm, T1 = 95 ms), acquired with identical acquisition parameters as in vivo. Corrections were applied for coil loading by measuring the formic acid containing phantom as external reference during each acquisition. Furthermore, different distances between coil and liver tissue were modelled by acquiring glycogen spectra with the glycogen phantom positioned at a range of distances. All liver spectra were processed using a custom-written Matlab script with in house established prior knowledge and the region of 100 ± 3 ppm was integrated.
Results
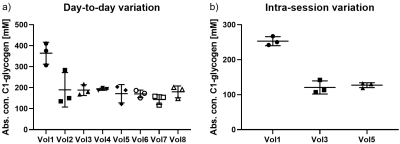
Figure 1 displays the variation of hepatic C1-glycogen concentrations for the day-to-day (three measurements within a week) and intra-session (with repositioning) study, respectively. Table 1 summarizes the corresponding coefficients of variation (CVs) as well as the absolute concentrations of hepatic glycogen for the particular sessions. The five volunteers, measured an additional fourth time after three months showed no changed C1-glycogen concentration with time compared to the group measured three times within a week (211 ± 76 mM vs. 200 ± 68 mM).Discussion and Conclusion
Non-localized 13C-MRS allows robust detection of absolute hepatic glycogen concentration by the phantom-replacement method within a reasonable time frame. Good reproducibility (CV < 22%) is shown when using an adiabatic pulse and a dual-tuned quadrature coil and determining the metabolites in healthy volunteers on another day or even after three months do not introduce a greater additional variation (17.8 % and 21.9 %). Using a quadrature surface coil allows the detection of C1-glycogen even in overweight and obese people (max. BMI = 32.4 kg/m2). The similarity of the obtained hepatic glycogen concentrations, compared to literature3 shows the robustness of the phantom-replacement method for absolute quantification.Acknowledgements
No acknowledgement found.References
[1] Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Braz arch biol technol. 2004; 53:3048–3056
[2] Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995; 95:783–787
[3] Buehler T, Bally L, Dokumaci AS, Stettler C, Boesch C. Methodological and physiological test-retest reliability of 13C-MRS glycogen measurements in liver and in skeletal muscle of patients with type 1 diabetes and matched healthy controls. NMR Biomed. 2016; 29:796–805
Figures

Table 1: Results of the day-to-day and intra-session study. Day-to-day and intra-session variability (CVs) and C1-glycogen levels in mM for the day-to-day and intra-session study. Concentration levels are reported as mean ± SD.