3688
Localized 31P Magnetic Resonance Spectroscopy of Normal Human Kidneys at 3T1Department of Radiology, University of Texas Southwestern Medical Center, DALLAS, TX, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, DALLAS, TX, United States, 3Novartis Institutes for BioMedical Research, Cambridge, MA, United States
Synopsis
Keywords: Spectroscopy, Kidney, Phosphorous (31P) MR spectroscopy (MRS), healthy volunteers
Phosphorous (31P) MR spectroscopy (MRS) can measure high energy phosphate metabolism non-invasively in vivo, which can provide metabolic insights into kidney pathophysiology. However, obtaining spectra from healthy volunteers for reference is challenging. In this study, we investigate the feasibility of localized 31P MRS in kidneys of healthy volunteers with a 3T clinical MR scanner. 31P spectra were successfully obtained in 10 healthy volunteers with metabolite ratio calculated to provide information about the energy metabolism.
Introduction
Human kidneys are metabolically active and utilize high levels of ATP for electrolyte balance, acid-base homeostasis, and nutrient reabsorption. Progressive loss of ATP and in turn increase of inorganic phosphorus are associated with the development of various kidney diseases including acute kidney injury, chronic kidney disease, and glomerular nephropathy. Hence detecting this high energy phosphate metabolism non-invasively in vivo can provide metabolic insights into kidney pathophysiology. Phosphorous magnetic resonance spectroscopy (31P-MRS) can directly measure the high-energy phosphates from metabolites such as phosphocreatine (PCr), inorganic phosphate (Pi), and adenosine triphosphate (ATP), with reported application in in-vitro renal metabolism.[1] However, obtaining spectra from healthy volunteers for reference is challenging, due to large distance between kidney and surface coil. Thus, the goal of our proof of principle study is to investigate the feasibility of localized 31P MRS in kidneys of healthy volunteers.Methods
Subjects: The study was performed in 10 healthy volunteers (mean age, 27 ± 3 years) with IRB approval using a 3T MRI scanner (Achieva, Philips Healthcare).Image Acquisition and Analysis: All volunteers were examined in the supine position. Several vitamin E capsules were placed on the subject’s back for localizing the kidneys and were then scanned using proton imaging with SShTSE. A 31P surface coil with a diameter of 14 cm was placed underneath the kidney using proton imaging and signal from Vitamin E as reference. Single voxel (typical size of 40 (AP) x 40 (LR) x 60 (FH) mm3) spectra were acquired using ISIS sequence (TR=2500 ms, NSA=1024, sample points=2048, spectral bandwidth=3000 Hz, scan time=45 mins). The acquired spectra were processed with jMRUI [2] software package with following steps: zero filling (4000 zeros), Lorentzian apodization (8 Hz), curve fitting with the AMARES algorithm [3]. The starting values were entered based on preprocessed spectrum and literature results with phosphomonoester (PME) at 7 ppm, inorganic phosphate (Pi) at 5.6 ppm, phosphodiester (PDE) at 3.1 ppm, phospho-creatinine (PCr) at 0.3 ppm, adenosine triphosphate-γ (ATP-γ) at -2.3 ppm, ATP- α at -7.4 ppm and ATP-β at -15.7 ppm. AMARES algorithm calculated the final intensities and positions of the individual peaks. For further evaluation, the following metabolite ratios were calculated based on their areas under the curve (AUC): PME/ Pi, PME/PDE, PDE/Pi, PME/ATP-α, PDE/ATP-α, PME/ATP-β, and ATP-γ/ATP-α.
Results and Discussion
Single voxel spectra were successfully obtained in all 10 healthy volunteers with a representative voxel position as shown in Figure 1. The voxel position covered most of the kidney while avoiding the muscle and fat to minimize signal contamination from PCr. The averaged 31P MR spectrum of kidneys from 10 healthy volunteers were fitted with AMARES algorithm (Figure 2). Although the acquisition voxel was carefully positioned for each MR spectrum, there was still some PCr contamination from the surrounding muscle due to subject respiration. Spectroscopic data and metabolite ratios were shown in Table 1. The resonance frequency for each metabolite was consistent with the existing literature, while we observed some differences in the reported metabolite ratios.Conclusion
In this study, we have demonstrated the feasibility of acquiring 31P MR spectrum of normal kidneys in vivo on a 3T clinical MR scanner. The metabolite ratios provide information about the energy metabolism [4] that can be potentially used to elucidate the pathophysiology of kidney disease. This will be particularly helpful in the evaluation of transplanted kidneys [5,6] since we anticipate that the reduced distance between 31P MRS surface coil and the transplanted kidney may provide improved performance. This may provide valuable information for patient management.Acknowledgements
This work was partly supported by Novartis Institutes for BioMedical Research. We would also like to thank our MR technologists (Daniel Tetrick and Corey Mozingo), research coordinators (Sydney Haldeman and Christina Craver) and all volunteers.
References
1. Sehr, P.A., et al., A model kidney transplant studied by phosphorus nuclear magnetic resonance. Biochemical and Biophysical Research Communications, 1977. 77(1): p. 195-202.
2. Stefan, D., Di Cesare, F., Andrasescu, A., Popa, E., Lazariev, A., Vescovo, E., Strbak, O., Williams, S., Starcuk, Z., Cabanas, M., van Ormondt, D., Graveron-Demilly. D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Measurement Science and Technology 20:104035 (9 pp), 2009. doi: 10.1088/0957-0233/20/10/104035
3. Vanhamme, L., van den Boogaart, A., Van Huffel, S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of Magnetic Resonance 129: 35-43, 1997.
4. Jafar M., Weis J. Phosphorus Magnetic Resonance Spectroscopy of Healthy Human Kidney in-situ at 3T. In: Proceedings of the ISMRM 29th Annual Meeting, 2021, Abstract number 2519.
5. Fiorina, P., Bassi, R., Gremizzi, C. et al. 31P-magnetic resonance spectroscopy (31P-MRS) detects early changes in kidney high-energy phosphate metabolism during a 6-month Valsartan treatment in diabetic and non-diabetic kidney-transplanted patients. Acta Diabetol 49 (Suppl 1), 133–139 (2012). https://doi.org/10.1007/s00592-012-0369-2
6. Fiorina P, Perseghin G, De Cobelli F, Gremizzi C, Petrelli A, Monti L, Maffi P, Luzi L, Secchi A, Del Maschio A. Altered kidney graft high-energy phosphate metabolism in kidney-transplanted end-stage renal disease type 1 diabetic patients: a cross-sectional analysis of the effect of kidney alone and kidney-pancreas transplantation. Diabetes Care. 2007 Mar;30(3):597-603. doi: 10.2337/dc06-1324. PMID: 17327327.
Figures
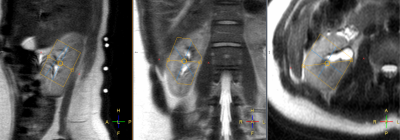
Figure 1: Typical single voxel planning of image guided localized 31P MR spectrum shown on sagittal, coronal and axial planes respectively. The voxel position covered most of the kidney while avoiding the muscle and fat. The four white circles on back of the subject in the sagittal plane (left) represent signals from the vitamin E capsules, used for positioning 31P surface coil.
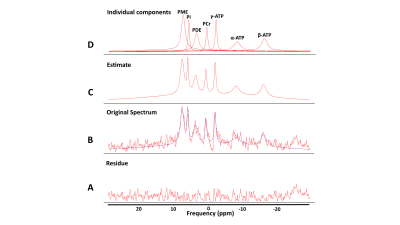
Figure 2: Curve fitting result with AMARES algorithm of averaged 31P MR spectrum of kidneys from 10 healthy volunteers. A) Residue signal between the original spectrum and the curve fitted estimate. B) The original spectrum obtained from 10 healthy volunteers’ fitted using AMARES algorithm and (C) the corresponding estimate. D) The individual peaks extracted from the whole spectrum.
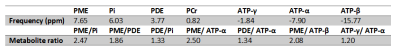
Table 1: Summarized spectroscopic data and metabolite ratios among healthy volunteers.