3687
Comparison of STEAM and sLASER to quantify acetylcarnitine at rest using long TE 1H-MRS in human skeletal muscle with a surface or birdcage coil at 7T1Scannexus (Ultra-High Field Imaging Center), Maastricht, Netherlands, 2Faculty of Health Medicine and Life sciences (FHML), Maastricht University, Maastricht, Netherlands, 3Department of Radiology and Nuclear Medicine, Maastricht University Medical Center (MUMC), Maastricht, Netherlands, 4Department of Cognitive Neuroscience, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 5Nutrition & Movement Sciences, Maastricht University, Maastricht, Netherlands, 6German Diabetes Center, Dusseldorf, Germany
Synopsis
Keywords: Spectroscopy, Muscle, Ultra-high field MRS, proton MR spectroscopy, Muscle
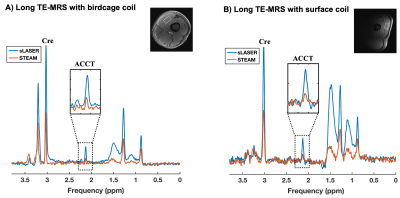
Long echo time 1H-MRS has been used to determine in vivo acetylcarnitine (ACCT) concentrations in the skeletal muscle. At ultra-high field (UHF), STEAM-based 1H-MRS was used for this purpose, in combination with a knee birdcage coil. However, STEAM suffers from an inherent 50% signal loss and a knee coil often does not fit around the upper leg in obese volunteers. Here, we demonstrated that sLASER, in combination with a surface coil, provides high signal-to-noise ratio (SNR) and can be used as an alternative method at 7T to detect physiologically low ACCT concentrations at rest in both lean and obese volunteers.Introduction
Proton magnetic resonance spectroscopy (1H-MRS) has been used to determine in vivo acetylcarnitine (ACCT) concentrations non-invasively in skeletal muscle1–4. ACCT is suggested to play a vital role in maintaining insulin sensitivity and glucose homeostasis5–7. Detection of ACCT is quite challenging with conventional short TE 1H-MRS as the ACCT resonance at 2.13 ppm is covered by strong (allylic) lipid resonances. Therefore, long TE 1H-MRS2 and T1 based editing techniques8 were developed previously to detect ACCT by minimizing the contamination of overlapping lipid resonances. Earlier studies mostly used PRESS at clinical field strength (3T)2,5,8 and only a few studies were performed at ultra-high field (UHF) to detect ACCT3,4. At UHF, STEAM is commonly used rather than PRESS as it requires minimal RF power, is less susceptible for J-coupling and shows minimal Chemical Shift Displacement Error (CSDE). However, the benefit of STEAM comes with an inherent 50% signal-to-noise ratio (SNR) reduction. While STEAM is recommended for the detection of short T2 metabolites, detection of ACCT is usually performed at long TE for relative lipid suppression and as ACCT has long T1 and T2 relaxation times3. Therefore, a semi-Localized by Adiabatic SElective Refocusing (sLASER) sequence is a good alternative, without the STEAM-based inherent signal loss and minimal CSDE artifact9,10. Indeed, the MRS expert consensus group recommended to apply sLASER for human brain MRS applications11, but it has not yet been explored in musculoskeletal MRS applications. The use of sLASER has the additional advantage of being less sensitive to B1 inhomogeneities which enables the use of surface coils. Previous studies at UHF used the combination of STEAM and a birdcage Knee RF coil to perform muscle MRS at 7T as it provides better B1 homogeneity3. However, with a knee coil, measurements in the upper leg can only be performed in lean individuals, while the main interest to study ACCT would be in individuals with metabolic disease, often overweight or obese. In that respect, surface coils would be more suitable to perform muscle MRS in both lean and obese volunteers. We aim to test whether the use of sLASER is indeed superior to STEAM in ACCT detection at 7T and whether it enables the use of a surface coil and detection in the thigh muscle at rest.Methods
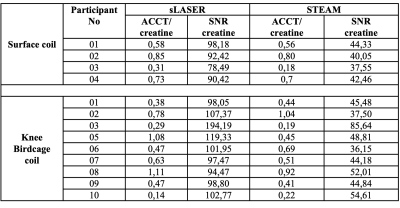
Experiments were performed in ten young healthy volunteers (m/f: 5/5; mean age: 25.5±6.5 years, mean BMI: 22.5±2.0 Kg/m2) on a 7T whole-body MR system (Magnetom, Siemens, Germany) using a 28-channel dedicated knee RF coil (QED, Mayfield Village, OH) and 1H/13C surface coil (MRI.TOOLS, Germany) in the feet-first supine position. All volunteers refrained from intensive exercise one day before the measurement and on the measurement day itself. Multislice scout MR images were acquired, and a small voxel was carefully placed in thigh muscle (vastus lateralis) in the left leg of volunteer. Standard B0 shimming was performed manually on the VOI after acquisition of B0 map (only for volume coil measurements) and voxel-based RF calibration performed prior to MRS acquisition. Spatially localized long TE 1H-MR spectra were acquired using both STEAM and sLASER (HS6, R20) sequence (obtained from CMRR, Minnesota) with the following parameters: Voxel=15x25x40 mm (15ml), TR/TE/TM=7000/350/30 ms, spectral bandwidth=4000 Hz, dummy scans=4, delta frequency=-2.5 ppm relative to water resonance, averages=32. No water suppression was applied. All the spectra were post-processed, and the target resonances were fitted using a custom-build MATLAB script. ACCT/creatine ratios were estimated after correcting for T2 relaxation effects as described previously3. As ACCT can fluctuate in-between two acquisitions, we used the creatine resonance, which is stable, to calculate SNR and compare the MR sequences as well as the sensitivity of the two RF coils. SNR was calculated as the ratio between the peak height of the creatine peak, relative to the standard deviation of a stable spectral region without signal.Results and discussion
As expected, the ACCT resonance at 2.13 ppm is clearly visible in both MR sequences while performing long-TE MRS as shown in figure 1. As expected, the calculated SNR for creatine was found to be higher with sLASER in all the volunteers when compared to STEAM as shown in table 1(129.5±3.6% higher when using the volume coil; 118±9.6% higher when using the surface coil). Using the surface coil, ACCT was clearly quantifiable, especially in combination with sLASER and the calculated SNR for the creatine peak was superior to the combination of STEAM with the knee coil, indicating the feasibility of using surface coil at 7T for in vivo ACCT measurements.Conclusion
sLASER provides high SNR which enabled the accurate quantification of physiologically low ACCT concentrations at rest in all volunteers with both RF coils. In combination with sLASER, a surface coil can be used and ACCT can also be determined in the upper leg in overweight and obese volunteers. The high SNR of sLASER together with the use of UHF is also beneficial to detect dynamic changes of ACCT, for example after exercise.Acknowledgements
We would like to acknowledge Edward J.Auerbach and Malgorzata Marjanska (CMRR, Department of Radiology, Minnesota) for the development of pulse sequences for the Siemens platform which were provided by the University of Minnesota under a C2P agreement. Also, David Palm for his help to recruit volunteers and all the volunteers for their kind participation in the study.References
1. Krššák M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2021;34(5):e4266.
2. Lindeboom L, Nabuurs CI, Hoeks J, et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124(11):4915-4925.
3. Klepochová R, Valkovič L, Gajdošík M, et al. Detection and Alterations of Acetylcarnitine in Human Skeletal Muscles by 1H MRS at 7 T. Invest Radiol. 2017;52(7):412-418.
4. Ren J, Lakoski S, Haller RG, Sherry AD, Malloy CR. Dynamic monitoring of carnitine and acetylcarnitine in the trimethylamine signal after exercise in human skeletal muscle by 7T 1H-MRS. Magn Reson Med. 2013;69(1):7-17.
5. Bruls YM, de Ligt M, Lindeboom L, et al. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine. 2019;49:318-330.
6. Hiatt WR, Regensteiner JG, Wolfel EE, Ruff L, Brass EP. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J Clin Invest. 1989;84(4):1167-1173.
7. Bruls YMH, Op den Kamp YJM, Phielix E, et al. L-carnitine infusion does not alleviate lipid-induced insulin resistance and metabolic inflexibility. PloS One. 2020;15(9):e0239506.
8. Lindeboom L, Bruls YMH, van Ewijk PA, et al. Longitudinal relaxation time editing for acetylcarnitine detection with 1 H-MRS. Magn Reson Med. 2017;77(2):505-510.
9. Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1-6.
10. Oz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901-910.
11. Öz G, Deelchand DK, Wijnen JP, et al. Advanced single voxel 1 H magnetic resonance spectroscopy techniques in humans: Experts’ consensus recommendations. NMR Biomed. 2020 Jan 10:e4236.
Figures