3676
Isolating the arterial blood volume change to probe fMRI spatial specificity1Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 2Computational Cognitive Neuroscience and Neuroimaging, Netherlands Institute for Neuroscience, Amsterdam, Netherlands, 3Techna Institute, University Health Network, Amsterdam, Netherlands, 4MR-Methods group, MBIC, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 5Donders Institute for Brain, Cognition and Behaviour, Donders Centre for Cognitive Neuroimaging, Radboud University Nijmegen, Nijmegen, Netherlands, 6Erwin L. Hahn Institute for Magnetic Resonance Imaging, Essen, Germany
Synopsis
Keywords: fMRI, fMRI, high field, cerebral blood volume
BOLD fMRI is widely used in neuroscience, but has limited spatial specificity while alternative approaches based on tissue cerebral blood volume (CBV) change have limited sensitivity. Recently, arterial CBV change (Arterial Blood Contrast; tracked through magnetization-transfer) was suggested as an fMRI mechanism, but its localization and sensitivity remain unexplored, since efficiently isolating the arterial CBV is challenging. Here, we combine temporally-efficient saturation with center-slice-out readouts and BOLD-correction to isolate arterial CBV for high-resolution 7T human fMRI. Our novel sequence shows much-improved specificity compared to BOLD and similar sensitivity to VASO, suggesting Arterial Blood Contrast to be a promising fMRI approach.Introduction
fMRI allows imaging the neural underpinnings of behavior in-vivo and has seen therefore widespread application1. fMRI typically uses deoxyhemoglobin as an endogenous contrast agent (BOLD), which biases spatial sensitivity towards vein-rich areas, like the pial-surface, complicating high-resolution applications2. Approaches targeting cerebral blood volume (CBV) changes in the tissue, like Vascular-Space-Occupancy (VASO), may localize neural activity better3, but are less sensitive. Arterial Blood Contrast (ABC) is a recently-introduced technique in humans4,5 that induces an arterial-weighted CBV signal6 facilitated by preferential tissue-suppression through magnetization-transfer7. ABC is expected to be more sensitive to microvasculature than BOLD, resulting in higher spatial specificity8. Isolating the arterial component to examine its localization and sensitivity is, however, not trivial due to BOLD contamination and SAR limitations, so the possible benefits of ABC remain unexplored. In this study, we develop a method to isolate ABC in high spatiotemporal resolution and examine its localization and specificity.Methods
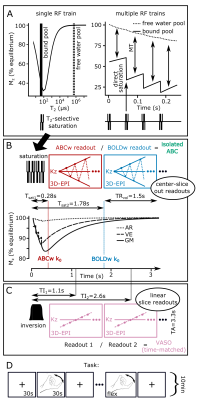
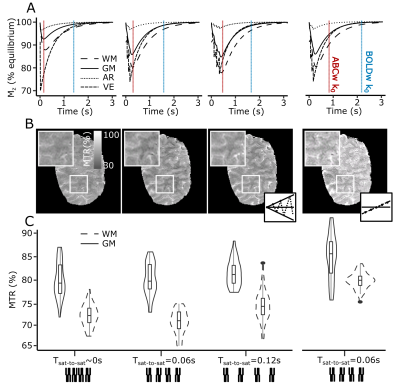
5 participants (4 male) were scanned in a Philips Achieva 7T with a 2Tx/32Rx whole-head coil (Nova-Medical). A T2-selective, rectangular, on-resonance phase-modulated pulse-train (B1=12μT) was employed to selectively saturate the bound-pool (Fig.1A-left). The bound-pool was allowed to cross-relax to the free-water-pool for 60ms before pulse train repetition, to maximize MT-weighting (4 pulse-trains; Fig.1A-right). Following the pulse-trains, two pairwise-interleaved 3D-EPI readouts were acquired (FOV=140x141x20mm3, voxel-size=0.8x0.8x1.5mm3, TE/TR/TAvolume/TAtotal=19/67/1500/3300ms, flip-angle=20o, SENSEy/z=2.7/1, partial-Fouriery=0.7). The 3D-EPIs were acquired in a center-slice-out fashion, so that the ABC k0 occurred at the maximum gray-matter saturation, while the BOLD-only image was acquired close to the longitudinal equilibrium (Fig.1B). This saturation difference allowed isolating the ABC signal through BOLD normalization (isolated-ABC=ABC/BOLD).Coupled-compartment Bloch simulations were performed for different pulse-train-to-pulse-train times (0, 60ms and 120ms) with literature T1/T2/lipid-fraction values for grey-matter, white-matter, veins and arteries9,10. These simulations were experimentally confirmed in 1 participant.
All participants performed a hand-flexing task (ON=30s, OFF=30, run-duration=10min), while the isolated-ABC sequence was recorded. For 3 participants, a temporally-matched VASO scheme (TE/TR/TI1/TI2/TAtotal=19/67/1100/2550/3300ms) was also recorded, achieved by shifting the first 3D-EPI k0 to the blood-nulling point by reordering the 3D-EPIs to a linear-slice-readout. The VASO and isolated ABC task runs were acquired in a counterbalanced fashion. The field-of-view was placed in the left primary motor cortex.
FMRI data were motion-corrected and a GLM (finger-tapping>rest) was fitted (FSL6.0.1). Gray-matter cortical depth profiles were extracted from a manually-drawn M1 ROI (LAYNII11).
Results
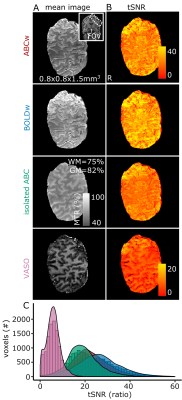
Cross-compartment Bloch simulations predicted maximal gray-matter, white-matter and venous saturation for this pulse-train for Tsaturation-to-saturation=60ms (Fig.2A). Pilot data agreed with these simulations (grey/white-matter saturation/contrast in Fig.2B-C). Acquisition without a saturation-to-saturation delay (Fig.2 left-column) or without a center-slice-out scheme (Fig.2 right-column) reduced the resulting MT-weighting.Magnetization-transfer-ratio (MTR) images with good image quality and gray-matter/white-matter contrast (MTRGM=82%, MTRWM=75%) could be extracted from the ABC/BOLD division (Fig.3A). The saturation-train reduced temporal-SNR (ABC: mean(sd)=21.8(8.4)) compared to BOLD: (mean(sd)=25.7(9.9)). The isolated-ABC had higher temporal-SNR (mean(sd)=18.6(7.1) than the time-matched VASO (mean(sd)=5.7(3); Fig.3B-C).
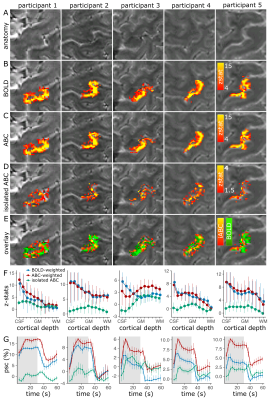
In all participants, a response localized to the M1/S1 gray-matter was found using the isolated-ABC, while BOLD responses were pial-surface-centred (Fig.4A-E). A cortical-depth analysis confirmed a much-reduced pial-surface-bias in isolated-ABC (Fig.4F). The isolated-ABC peristimulus-response-function was temporally similar to BOLD (Fig.4G).
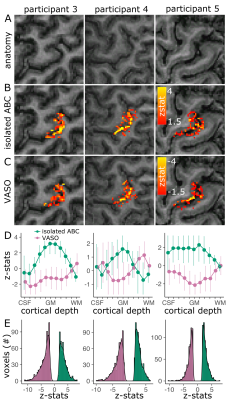
The isolated-ABC response was visually similarly localized (Fig.5A-C) and had a similar cortical depth response to VASO (Fig.5D). In M1, the ABC and VASO z-stat distributions were similar across participants, implying similar sensitivity (Fig.5E).
Discussion
Increasing the fMRI spatial specificity within the human cerebral cortex is an important neuroscientific target. Here, we developed a novel sequence with much-improved fMRI localization compared to BOLD.In the current (limited) research on magnetization-transfer-weighted fMRI5,6, contrast is created by rapidly-repeating pulse-trains, taking advantage of the slow longitudinal relaxation to retain saturation despite SAR-restrictions at higher-fields8. Here, we sparsely applied an optimized pulse-train combined with a center-slice-out readout to efficiently achieve MT-weighting. This facilitated straightforward BOLD-correction by interleaving an additional readout at the longitudinal equilibrium, thus isolating the ABC signal. The isolated-ABC showed much-reduced pial-surface bias compared to BOLD, in accordance with the hypothesized microvasculature dominance of arterial CBV. Our results replicate a similar gray-matter specific response found using magnetization-transfer fMRI in cats4.
While the current isolated-ABC implementation had similar specificity/sensitivity to VASO, the contrast-to-noise in our acquisition strategy can be further increased through longer pulse-trains, potentially increasing sensitivity in the future. Furthermore, the ABC signal is not sensitive to inflow effects and it facilitates arbitrary readouts (i.e. no blood-nulling requirement), resulting in a flexible CBV method applicable across the brain. The sensitivity of our approach to the magnetization exchange between intra- and extravascular spaces remains to be examined, but potentially our method can be expanded towards quantitative arterial CBV measurements in the future7.
The current isolated-ABC relied on hard-rectangular pulse-trains, trading off B1/B0 inhomogeneity sensitivity for efficient saturation. PTX-enabled, off-resonance pulse-trains may offer a better tradeoff for high-field application across the brain.
Conclusion
Overall, we demonstrate that the proposed isolated-ABC yields a consistent and highly-localized fMRI signal in humans at 7T. ABC-fMRI may be a useful tool for high-resolution human fMRI at high fields.Acknowledgements
This research was supported by a NWO VIDI grant to Wietske van der Zwaag (TTW VI.Vidi.198.016). Benedikt Poser is funded by a NWO VIDI grant (16.Vidi.178.052) and a National Institute for Health grant (R01MH/111444)References
1. Glover, G.H., Overview of functional magnetic resonance imaging. Neurosurg Clin N Am, 2011. 22(2): p. 133-9, vii.
2. Uludag, K., B. Muller-Bierl, and K. Ugurbil, An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage, 2009. 48(1): p. 150-65.
3. Huber, L., et al., Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. Neuroimage, 2018. 164: p. 131-143.
4. Kim, T. and S.G. Kim, Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab, 2011. 31(5): p. 1211-22.
5. Schulz, J., et al., Arterial blood contrast (ABC) enabled by magnetization transfer (MT): a novel MRI technique for enhancing the measurement of brain activation changes. 2020: Biorxiv.
6. Pfaffenrot, V. and P.J. Koopmans, Magnetization transfer weighted laminar fMRI with multi-echo FLASH. Neuroimage, 2022. 264: p. 119725.
7. Kim, T., K. Hendrich, and S.G. Kim, Functional MRI with magnetization transfer effects: determination of BOLD and arterial blood volume changes. Magn Reson Med, 2008. 60(6): p. 1518-23.
8. Priovoulos, N., et al., Submillimeter Arterial Blood Contrast fMRI at 7T, in Proc. Intl. Soc. Mag. Reson. Med. 29. 2021: Paris.
9. van Gelderen, P., X. Jiang, and J.H. Duyn, Rapid measurement of brain macromolecular proton fraction with transient saturation transfer MRI. Magn Reson Med, 2017. 77(6): p. 2174-2185.
10. Markuerkiaga, I., M. Barth, and D.G. Norris, A cortical vascular model for examining the specificity of the laminar BOLD signal. NeuroImage, 2016. 132: p. 491-498.
11. Huber, L.R., et al., LayNii: A software suite for layer-fMRI. Neuroimage, 2021. 237: p. 118091.
Figures