3670
Pushing limits of spatial resolution in 3D EPI for fMRI on the NexGen 7T scanner1Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 4Radiology, University of California, San Francisco, CA, United States, 5San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States
Synopsis
Keywords: fMRI, Neuroscience
Resolution in functional imaging can be increased by limiting the Field of View (FOV) for the image, increased acceleration and faster imaging using specialized gradient systems. Here we combine the high performance gradients of the NexGen 7T scanner with limited FOV imaging to collect ultra-high resolution fMRI in the occipital pole. Isotropic resolutions of 0.35mm are demonstrated using 3D EPI.Introduction
Two categorical approaches can be used to increase resolution in fMRI. The zoomed, inner volume approach restricts the signal volume using spatially selective RF pulses1. A second approach places the image plane or 3D volume at an oblique angle through the head to reduce the extent of the brain on the in-plane phase axis2. Both of these methods work by reducing the image FOV allowing finer sampling while avoiding aliasing of signal outside the FOV. These techniques have successfully been used for mesoscale imaging at 7T using conventional gradient systems1,2.These approaches can reach even higher resolution and faster data sampling rates using high performance gradients and higher in-plane parallel imaging accelerations. Here we evaluate the application of reduced FOV imaging with a high performance gradient system, the Impulse gradient coil of the MAGNETOM Terra Impulse edition NexGen 7T scanner. A 3D EPI acquisition with its imaging volume oriented coronally through the occipital pole was used to image the visual cortex at very high resolution.
Methods
MRI Scanner: Higher resolution can be achieved without lengthening the echo spacing or the echo train length using the high performance “Impulse” gradient (Siemens Healthcare, Erlangen, Germany) capable of a slew rate of 900 T/m/s and a maximum gradient of 200 mT/m. MRI data acquired using a 96 ch Rx, 16 ch Tx coil (MR CoilTec)3.Acquisition parameters: A segmented 3D-EPI4 sequence was used for ultra-high resolution BOLD imaging. The sequence protocol that was used for functional data acquisitions consisted of 18 partitions, GRAPPA 1-3 (with 5000 fold regularization), TE 19-23ms, pFourier 6/8 with POCS reconstruction with 8 iterations, in-plane matrix 374-462, 2-6 segments across kz planes, slice thickness 0.35mm, and in-plane resolution 0.35mm x 0.35mm. These scan protocols were initially developed for high-resolution VASO imaging of V15, and to achieve 0.35mm resolution the phase encoded FOV was restricted to 50% of the readout FOV and slices were acquired coronally across the occipital pole as has been applied previously 2. To maintain SNR for functional imaging at such high resolutions, NORDIC was used6. BOLD activations were assessed using a flashing checkerboard task. Data was motion corrected using AFNI, and activations in V1 were assessed using mrTools.
Results
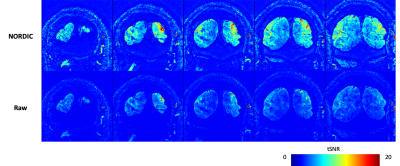
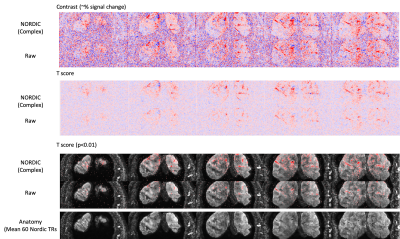
Figure 1 shows the spatial coverage available using the ultra-high resolution 3D EPI protocol. The imaging slab covered the tip of the occipital pole, covering foveal representations in V1 and and dorsal V2/V3 (Fig 1A). The reduced phase encoding FOV in the RL direction led to some aliasing of the scalp and skull in deeper slices (Fig 2B), although this did not overlap with our region of interest. Although the raw images are noisy, the use of NORDIC denoising leads to improved images even in individual TRs. NORDIC also improves the tSNR in the resultant images (Figure 2), and improves the resulting activation statistics while leaving the underlying signal changes unaffected (Figure 3).Discussion
Imaging at such ultra-high resolutions presents significant challenges. The small voxel size, and in particular the thin slices required, lead to insufficient SNR in 2D imaging. In addition, the slice profiles forsuch submillimeter regimes are challenging to achieve, increasing the risk of cross talk when using multiple contiguous slices. Segmented 3D EPI approaches increase the signal used in the 3D Fourier Transform for each image, increasing noise averaging and raising SNR, making them an attractive option for such submillimeter applications. The use of a 3D volume also allows contiguous slices without slice profile errors given the larger slab volume thickness compared to 2D thin slices. The use of denoising techniques such as NORDIC6 allow further increases in SNR.The second challenge is the echo train length. Using standard gradients, the long echo trains needed for high resolution lead to later TE, signal loss, increased PSF loss of true resolution and distortions. Therefore limiting the imaging FOV, and hence the overall echo train length, is an approach that has been used frequently. By combining this with the advanced gradients available on the NexGen scanner, we are able to reduce the echo spacing and push to the highest resolutions seen so far in human fMRI using EPI. In addition, the skipped-CAIPI 3D EPI sequence used here allows acceleration and in-plane segmentation which can be optimally combined to even further shorten the echo train for high resolution imaging with an optimized TE. This allows 0.35mm isotropic imaging of V1 (Figures 2 and 3).
Conclusion
The NexGen 7T demonstrates the efficacy of targeted ultra-high resolution acquisitions useful for studying specific brain regions. With further optimization of acceleration, segmentation and processing, larger volumetric brain coverage at ultra-high resolution is also possible.Acknowledgements
This project is supported by the NIH BRAIN Initiative (R01MH111444, U01EB025162), 1R44MH129278. Samantha Ma is supported by Siemens Medical Solutions USA, Inc.
We thank Rüdiger Stirnberg and Tony Stöcker for the use of the Skipped-CAIPI 3D EPI sequence, and we thank Renzo Huber for his help in setting up the initial protocol this work was based on.
References
1. Feinberg DA, Harel N, Ramanna S, Ugurbil K, Yacoub E, Sub-millimeter Single-shot 3D GRASE with Inner Volume Selection for T2 weighted fMRI applications at 7 Tesla, p2373, ISMRM 2008, Toronto
2. Feinberg, D. A., Vu, A. T., & Beckett, A. (2018). Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI. Neuroimage, 164, 155-163. doi:10.1016/j.neuroimage.2017.02.020
3. Gunamony S, Müller R, McElhinney P, Williams SN, Groß-Weege N, Weiskopf N, Möller HE, Feinberg DA (2021) “A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner” ISMRM
4. Stirnberg, Rüdiger, and Tony Stöcker. 2021. “Segmented K-space Blipped-controlled Aliasing in Parallel Imaging for High Spatiotemporal Resolution EPI.” Magnetic Resonance in Medicine 85(3):1540–51. doi: 10.1002/mrm.28486.
5. Feinberg, D.A, S. Torrisi,S A.J.S. Beckett, R. Stirnberg, S. Stocker, P. Ehses, and R. Huber. Sub-0.1 microliter CBV fMRI on the Next Generation 7T scanner. in Proceedings of the 29th Annual Meeting of ISMRM. 2022.
6. Vizioli, L., Moeller, S., Dowdle, L., Akçakaya, M., De Martino, F., Yacoub, E., & Uğurbil, K. (2021). Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nature communications, 12(1), 1-15.
Figures