3669
Combining the benefits of 3D acquisitions and spiral readouts for VASO fMRI at UHF1Maastricht University, Maastricht, Netherlands, 2Polytechnic University of Turin, Turin, Italy, 3University-Paris-Saclay, CEA, CNRS, BAOBAB, NeuroSpin, Gif-sur-Yvette, France
Synopsis
Keywords: fMRI, High-Field MRI, Pulse Sequence Design
VASO fMRI can provide beneficial localization specificity and quantifiability compared to the commonly used BOLD contrast. Previous work has also shown the benefits of using spiral readouts compared to Cartesian EPI. In this work, we employ 3D stack-of-spirals readouts and compare it with the current state of the art 3D EPI readouts for VASO fMRI. The sequence implementation is done using Pulseq, images were reconstructed with MRIReco.jl; functional analysis with an openly available pipeline. We find that a VASO tSNR improvement of a factor of 2 over EPI is achieved using the proposed spiral implementation.Introduction
VAscular Occupancy (VASO)1 can outperform the most commonly used BOLD techniques with respect to its physiological interpretability and its localization specificity; this is especially the case at ultra-high fields and at high spatial resolution (<1.5 mm)2. Some of the main limitations of VASO are BOLD contamination (which affects the VASO contrast), limited detection sensitivity, and temporal sampling efficiency. For the BOLD contamination, it has been shown that a BOLD-corrected VASO image can be obtained by means of dynamic division of concomitantly acquired control images3. To overcome the limitations set by low temporal sampling efficiency, previous work has suggested combining the efficiency of spiral k-space sampling with simultaneous multi-slice acquisitions4. For high-resolution fMRI, however, 3D readouts can be advantageous over 2D readouts2. The purpose of this study is to combine the benefits of 3D acquisitions and the efficiency of spiral for VASO fMRI. This is possible using a 3D stack-of-spirals readout which has previously been advocated for BOLD fMRI5. Here, we demonstrate that fMRI with VASO contrast can be obtained using spirals, allowing for shorter echo times to improve temporal SNR, remove BOLD contamination and reduce the effective acquisition time.Methods
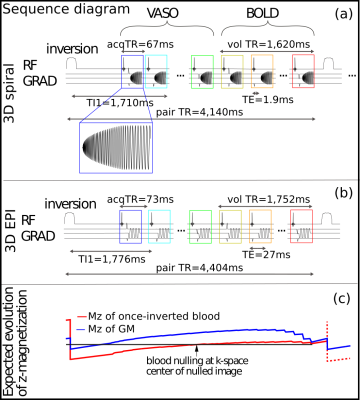
A 3D stack-of-spirals Slab-Selective Slab-Inversion (SS-SI VASO) sequence was implemented using Pulseq6, a framework that allows for rapid prototyping and implementation of sequences on the scanner. For BOLD contamination correction, a BOLD-weighted control image was acquired right after the VASO one2. A 10 ms TR-FOCI inversion pulse7 was applied 900 ms before the first excitation pulse of the spiral-out readout train; fat suppression was achieved by means of a 5 ms Gaussian pulse; the spiral trajectory was designed to minimize its duration within gradient constrains8. The sequence used in this work is shown in Figure 1. In vivo data were acquired on a 7T scanner (Siemens Healthineers) with 32ch headcoil (Nova Medical), visual checkerboard stimuli was presented while the subjects performed a fingertapping motor task (block-design, 33s/33s ON/OFF, 9 blocks). Spiral acquisition parameters were: FOV=192x192x24 mm3, nominal resolution=0.8x0.8x1.0 mm3, 24 kz partitions with linear encoding order, TRvol=1622 ms, TE=1.9 ms, TI1=1660 ms. The spiral readout was designed with variable density (α=1.6) and a factor 3 in-plane undersampling resulting in a spiral duration of 53 ms. Cartesian 3D EPI9 VASO with matched parameters and same task was acquired for comparison: matrix size [240 240 24], 1.02 ms echo spacing, bandwidth 1096 Hz/px, GRAPPA 3 and 6/8 partial Fourier resulting in 63 ms readout duration, linear kz enconding, selection of minimum TE/TR = 27/1754 ms. The resulting effective TR for the spiral and EPI sequences are 4140ms and 4404 ms, respectively. EPI data were reconstructed using the vendor software on the scanner. The spiral reconstruction was performed in the open-source software MRIReco.jl10 using CG-SENSE and time-segmented off-resonance correction using the nominal gradient trajectory; coil sensitivity and B0 maps were taken from a separately acquired multi-echo 2D GRE scan. fMRI analysis was done in the openly available VASO pipeline [https://github.com/layerfMRI] consisting of motion correction, BOLD correction and conventional quality measures (tSNR, mean timecourse image, GLM statistics).Results and Discussion
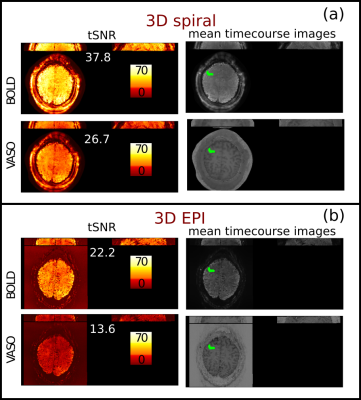
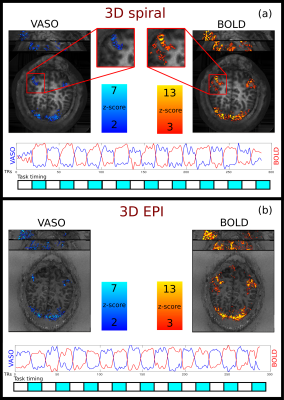
Figure 2 shows tSNR maps and mean timecourse images of the spiral and Cartesian data. In the thermal noise dominated regime of the high-resolution VASO, the spiral approach outperforms the EPI sampling by a factor of 2 in tSNR. This is expected from cumulative gains of improved signal sampling, including shorter echo times with reduced T2*-decay. Figure 3 shows the activation maps obtained from the spiral and EPI acquisitions it can be seen that the spatial distribution of the activation pattern obtained with the current implementation is comparable to the current state of the art 3D EPI sequence, confirming that the spiral acquisition effectively captures CBV and BOLD changes.Summary and Conclusion
In this work, we have shown that a fast implementation of a VASO fMRI with a 3D stack-of-spirals readout can be achieved using open source and freely available programs such as Pulseq, MRIReco.jl and analysis pipeline. The higher tSNR achieved with the spiral readout shows the potential of spiral VASO fMRI. The tSNR improvement over 3D EPI, however, is unmatched by a z-score increase, which remains to be investigated. Further work will focus on implementing acceleration with controlled aliasing in the kz direction, reconstruction speed-up and dynamic field monitoring. Here we demonstrate that spiral readouts are promising especially in applications where there is a need for short TE, such as mesoscopic functional experiments at higher fields such as 9.4T and 11.7T where T2* is shorter.Acknowledgements
This work has been funded by the H2020 FET-Open AROMA grant agreement no. 88587.
Benedikt Poser is also funded by the NWO VIDI grant 16.Vidi.178.052.
Renzo Huber was funded by the NWO VENI project 016.Veni.198.032.
References
1. Lu, H., Golay, X., Pekar, J. J., & Van Zijl, P. C. (2003). "Functional magnetic resonance imaging based on changes in vascular space occupancy". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 50(2), 263-274.
2. Huber, L., Ivanov, D., Handwerker, D. A., Marrett, S., Guidi, M., Uludağ, K., … Poser, B. A. (2018). “Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications.” NeuroImage, 164, 131–143.
3. Huber, Laurentius et al. (2014). “Slab-Selective, BOLD-Corrected VASO at 7 Tesla Provides Measures of Cerebral Blood Volume Reactivity with High Signal-to-Noise Ratio.” Magnetic Resonance in Medicine 72(1): 137–48.
4. Zahneisen, B., Poser, B. A., Ernst, T., & Stenger, A. V. (2014). "Simultaneous Multi-Slice fMRI using spiral trajectories". NeuroImage, 92, 8-18.
5. Hu, Y., & Glover, G. H. (2007). "Three‐dimensional spiral technique for high‐resolution functional MRI". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 58(5), 947-951.
6. Layton, K. J., Kroboth, S., Jia, F., Littin, S., Yu, H., Leupold, J., ... & Zaitsev, M. (2017). "Pulseq: a rapid and hardware‐independent pulse sequence prototyping framework". Magnetic resonance in medicine, 77(4), 1544-1552.
7. Hurley, A. C., Al‐Radaideh, A., Bai, L., Aickelin, U., Coxon, R., Glover, P., & Gowland, P. A. (2010). "Tailored RF pulse for magnetization inversion at ultrahigh field". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 63(1), 51-58.
8. Lustig, M., Kim, S. J., & Pauly, J. M. (2008). A fast method for designing time-optimal gradient waveforms for arbitrary k-space trajectories. IEEE transactions on medical imaging, 27(6), 866-873.
9. Poser, B. A., Koopmans, P. J., Witzel, T., Wald, L. L., & Barth, M. (2010). "Three dimensional echo-planar imaging at 7 Tesla". Neuroimage, 51(1), 261-266.
10. Knopp, T., & Grosser, M. (2021). MRIReco. jl: An MRI reconstruction framework written in Julia. Magnetic resonance in medicine, 86(3), 1633-1646.
Figures