3638
The feasibility of distinguishing HSPN from HSP in children using intra-voxel incoherent motion diffusion weighted imaging1The First Affiliated Hospital of Henan University of CM, Zhengzhou, China, 2Philips healthcare, Beijing, China
Synopsis
Keywords: Kidney, Kidney
Currently, the therapeutic significance of IVIM-DWI has been examined in numerous kidney illnesses such as transplanted kidney, renal tumor, and renal failure; however, the application in purpura nephritis is uncommon. As a result, the purpose of this study was to investigate the clinical feasibility of IVIM-DWI in differentiating purpura nephritis (HSPN) from allergic purpura (HSP) in children by using D and K values obtained from a small-field DWI sequence with respiratory triggerIntroduction
Intravoxel incoherent motion diffusion imaging (IVIM-DWI) can indicate hyperperfusion and excessive water molecule metabolism in the kidney [1] and aid in the assessment of widespread renal damage. IVIM-DWI has been investigated in a variety of kidney disorders, including chronic kidney disease[2, 3], diabetic nephropathy[4, 5]. However, to the best of our knowledge, there have been few studies on IVIM-DWI imaging in purpura nephritis. As a result, the purpose of this study was to investigate the clinical feasibility of IVIM-DWI in distinguishing HSPN from HSP using D and K values obtained from a small-field DWI sequence with respiratory trigger to acquire high-resolution coronal renal diffusion images.Method
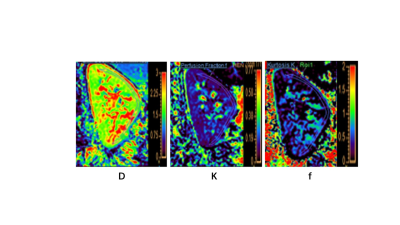
This study included 9 children with HSPN, 8 children with HSP, and 8 healthy volunteers (control). After anatomical imaging for respiratory-triggered left-sided renal, IVIM-DWI was performed on a 3.0T MR scanner (Ingenia CX, Philips Healthcare, The Netherlands) using renal transverse and coronal T2W scans. Advanced diffusion processing (ISP, Philips Healthcare, the Netherlands) was used to analyze the IVIM maps, and b-value maps affected by respiratory motion were deleted. The biexponential model was used to produce the D-map, D*-map, F-map, automatically. The basic IVIM + kurtosis model was used to generate the K-map. The area of interest (ROI) was drawn manually. The Kolmogorov-Smirnov and Levene methods were used to assess group data for normality and chi-squared. The mean standard deviation was used to characterize measurements that corresponded to the normal distribution. To compare group differences in D values, D*, and K values, an independent samples t-test was utilized. Spearman correlation analysis was used to investigate potential relationships between the MRI indices, as well as between MRI indices and biochemical markers (24h proteinuria and crescent). Scatter plots and linear regression techniques were used to calculate correlation coefficients. ROC analysis was used to determine diagnostic performance and indicator threshold values.Results
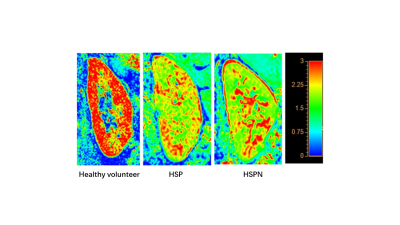
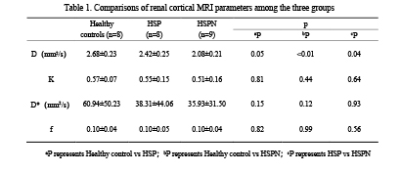
D values in renal cortex were lower in the HSP group than in the control group (P > 0.05), and significantly lower in the HSPN group than in both the control and HSP groups (P 0.05). In all cases, renal cortical D levels were positively correlated with K values. The AUC distribution of D values for recognizing HSP and HSPN was 0.802, with a threshold of 2.06 mm2/s and a specificity of 100%.Discussion and Conclusion
D values were observed to be considerably lower in the HSPN group compared to both the control and HSP groups in this study, which may be related to HSPN immune complex deposition and varying degrees of thylakoid hyperplasia causing limited diffusion of water molecules in the kidney. As a result, IVIM-DWI may be utilized to assess renal damage in HSPN, which is consistent with earlier research[6]. Renal cortical D values, in particular, can be utilized to differentiate HSPN kidneys from HSP kidneys.We established in this preliminary study that IVIM-DWI was helpful for non-invasive evaluation of renal dysfunction in children with HSPN, and renal cortical D values might be a viable indicator for distinguishing HSPN from HSP kidneys.
Acknowledgements
No acknowledgement found.References
[1] Franca Mart-Bonmatil, Alberich-Bayarria, et al. Evaluation of fibrosis and inflammation in diffuse liver disease using intravoxel incoherent motion diffusion-weighted MR imaging[J]. Abdom Radiol,2017,42(2):468-477
[2] Sukowska K, Palczewski P, Furmanczyk-Zawiska A, et al. Diffusion weighted magnetic resonance imaging in the assessment of renal function and parechymal changes in chronic kidney disease: a preliminary study[J]. Ann Transplant, 2020, 25:e920232
[3] Feng YZ, Chen XQ, Yu J, et al. Intravoxel incoherent motion(IVIM) at 3.0 T: evaluation of early renal function changes in type 2 diabetic patients[J]. Abdom Radiol(NY),2018,43:2764-2773
[4] Zheng Z, Yan T, Jia J, et al. Assessment of Renal Pathological Changes in Lupus Nephritis Using Diffusion Weighted Imaging: A Multiple Correspondence Analysis. Kidney Blood Press Res. 2018;43(3):847-859. doi: 10.1159/000490333. Epub 2018 May 30.
[5] Liang P, Li S, Yuan G, et al. Noninvasive assessment of clinical and pathological characteristics of patients with IgA nephropathy by diffusion kurtosis imaging. Insights Imaging. 2022 Jan 29;13(1):18. [6] Lang ST, Guo J, Bruns A, Dürr M, Braun J, Hamm B, Sack I, Marticorena Garcia SR. Multiparametric Quantitative MRI for the Detection of IgA Nephropathy Using Tomoelastography, DWI, and BOLD Imaging. Invest Radiol. 2019 Oct;54(10):669-674.
Figures