3630
Morphological and Functional Evaluation of Diabetic Kidney Disease Cohorts by using T2-Weighted and Diffusion Weighted MRI1Department of Radiology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences,Guangzhou 510080, China, Guangzhou, China, 2Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China, Guangzhou, China, 3Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China, Guangzhou, China, 4Division of Nephrology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China, Guangzhou, China, 5Philips Healthcare Beijing Ltd., Shenzhen, China, 6Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, Berlin, Germany
Synopsis
Keywords: Kidney, Kidney
Diabetic Kidney Disease (DKD) is the leading cause of end-stage renal disease worldwide. Morphological evaluation of renal compartment volume changes and functional evaluation of renal ADC changes provide comprehensive insight into DKD disease progression. This study compares renal compartment volume changes including: 1) parenchyma volume ,2) pelvis volume, 3) whole kidney volume and 4) parenchyma volume percentage and the functional parameter ADC for DKD groups with different CKD staging. Our results demonstrate that cortical/medullary ADC, parenchyma volume and whole kidney volume are correlated with eGFR and suggest that the cortical ADC is a stronger predictor of eGFR in DKD.Introduction
With the prevalence of type 2 diabetes mellitus (DM), diabetic kidney disease (DKD) is the leading cause of end-stage renal disease worldwide with high morbidity and mortality(1-3). Mapping the apparent diffusion coefficient (ADC) and renal volume assessment are clinically meaningful tools for evaluating the renal micro- and macro- structure changes during DKD(4). Here the semi-automatic twelve-layer concentric objects (TLCO) technique is used for layer-specific assessment of ADC maps to provide a highly reproducible and reliable assessment of renal ADC in the cortex and medulla compared to the conventional region-of-interest method(5,6). This study compares morphological features of renal volume including the 1) parenchyma volume, 2) pelvis volume, 3) whole kidney volume, and 4) parenchyma volume ratio and functional renal MR parameter ADC that assessed with TLCO, among DKD groups with different CKD staging.Method
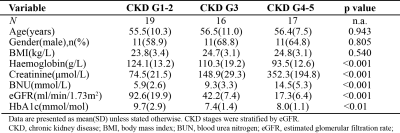
This cross-sectional study was approved by the local ethics review board of Guangdong Provincial People’s Hospital. Written informed consent was obtained from all subjects. Subject characteristics are presented in Figure 1. The DKD subjects were stratified by eGFR(calculated according to CKD-EPI) into 5 groups according to Kidney Disease: Improved Global Outcome of chronic kidney disease criteria(7,8). Due to the limited sample size, DKD groups are merged into mild DKD group (CKD G1-2), with eGFR > 60 ml/min/1.73m2, moderate DKD group (CKD G3), with 30 ml/min/1.73m2 < eGFR < 59 ml/min/1.73m2, and severe DKD group (CKD G4-5), with eGFR < 29 ml/min/1.73m2.MRI
MRI was conducted using a whole-body 3T scanner (SIGNA EXCITE, GE Healthcare) using the built-in body coil for signal excitation and an 8-channel RF torso array for signal reception. Respiratory triggering was used to avoid motion artifacts. For anatomical imaging, T2-weighted coronal fat-suppressed 2D multi-slice FSE imaging was implemented: TE=215ms, TR=15789ms, matrix=512×512, in-plane resolution=0.625×0.625mm2, slice thickness=3mm, slice gap=0.6mm. For DWI, coronal fat-suppressed 2D multi-slice single-shot spin-echo EPI was implemented: TE=60ms, TR=3000ms, matrix=96×96,in-plane resolution=1.25×1.25mm2, slice thickness=3mm, slice gap=0.6mm, b value=0 and 600 s/mm2.
Data analysis
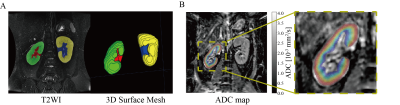
Figure 2 illustrates the post-processing of the T2WI and ADC map that was analyzed by using the TLCO approach.
Morphological evaluation of the kidney includes the assessment of 1) the volume of renal parenchyma, 2) the volume of the renal pelvis, 3) the volume of the whole kidney, and 4) the volume percentage of renal parenchyma (i.e. the volume of renal parenchyma divided by whole kidney volume). To measure the volume of renal parenchyma and pelvis, renal parenchyma and pelvis were manually labeled by the experienced radiologist on T2WI by using ITK-SNAP(9). Figure 2A illustrates the labeling of the renal parenchyma and pelvis, and the reconstructed 3D surface mesh. Following this segmentation, the volume was corrected by the body surface area (BSA) which is calculated according to: BSA(m2)=0.61×height(m) +0.0128×body weight(kg) – 0.1529 (10).
The middle coronal slice DWI data was analyzed by using Matlab scripts (Matlab 2018b). ADC map was calculated first followed by the TLCO approach which segmented the renal parenchyma into 12 layers with equal thickness. Mean ADC values were calculated for each layer (Figure 2B). The mean ADC obtained for the outer 6 layers was assigned to the ADC of the renal cortex (ADCcor), and the mean ADC value derived from the inner 6 layers was considered as the renal medulla ADC (ADCmed). ADCcor and ADCmed were compared among DKD groups. Layer-wise comparison among DKD groups was conducted.
Statistical analysis
Statistical analysis was conducted using SPSS (SPSS 26, IBM). The mean value of volume and ADC of the left and right kidneys were calculated and considered as the MR biomarkers for the subject. MR biomarkers were compared among groups using one-way ANOVA with post-hoc LSD or Kruskal-Wallis one-way ANOVA with post-hoc Bonferroni depending on data normality. Pearson correlation between MR biomarkers and eGFR was analyzed. P<0.05 was considered significant.
Results
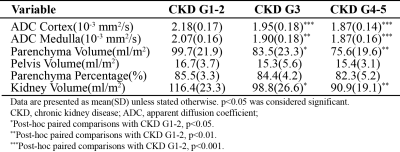
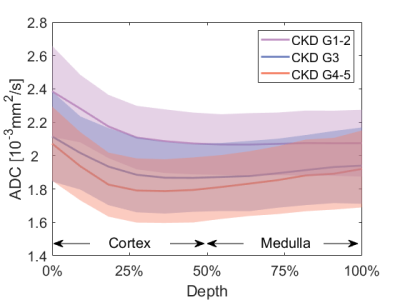
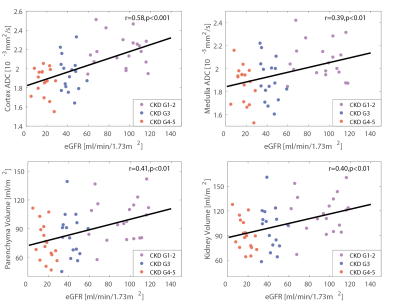
Figure 3 summarizes the MR biomarkers for the DKD group with different CKD staging. With the advanced CKD stages, ADCcor and ADCmed are significantly decreased for the moderate DKD and severe DKD groups compared to the mild DKD cohorts. This observation is confirmed by the layer-wise ADC comparison (Figure 4). Significant parenchyma volume and whole kidney volume reduction were observed for the moderate DKD group and severe DKD group versus the mild DKD group, while the pelvis volume and parenchyma volume percentage didn’t show significant changes. Pearson correlation analysis showed that ADCcor is moderately correlated with eGFR. ADCmed, parenchyma volume, and whole kidney volume are weakly to moderately correlated with eGFR (Figure 5).Conclusion
This study evaluated renal volume as a macro-morphological feature and renal water diffusion as a functional and micro-morphological feature of the kidney assessed by using the TLCO approach among DKD cohorts with different CKD staging. Our results demonstrate that ADCcor, ADCmed, parenchyma volume, and whole kidney volume are correlated with eGFR, and ADCcor is a stronger predictor of eGFR in DKD.Acknowledgements
noReferences
1. Zhang XX, Kong J, Yun K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J Diabetes Res 2020;2020:2315607.
2. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The Global Epidemiology of Diabetes and Kidney Disease. Adv Chronic Kidney Dis 2018;25(2):121-132.
3. Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME. Diabetic kidney disease. Nat Rev Dis Primers 2015;1:15018.
4. Makvandi K, Hockings PD, Jensen G, Unnerstall T, Leonhardt H, Jarl LV, Englund C, Francis S, Sundgren AK, Hulthe J, Baid-Agrawal S. Multiparametric magnetic resonance imaging allows non-invasive functional and structural evaluation of diabetic kidney disease. Clin Kidney J 2022;15(7):1387-1402.
5. Milani B, Ansaloni A, Sousa-Guimaraes S, Vakilzadeh N, Piskunowicz M, Vogt B, Stuber M, Burnier M, Pruijm M. Reduction of cortical oxygenation in chronic kidney disease: evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol Dial Transplant 2017;32(12):2097-2105.
6. Piskunowicz M, Hofmann L, Zuercher E, Bassi I, Milani B, Stuber M, Narkiewicz K, Vogt B, Burnier M, Pruijm M. A new technique with high reproducibility to estimate renal oxygenation using BOLD-MRI in chronic kidney disease. Magn Reson Imaging 2015;33(3):253-261.
7. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80(1):17-28.
8. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158(11):825-830.
9. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31(3):1116-1128.
10. Xia X, Huang N, Li B, Li Y, Zou L, Yuan D, Huang B, Bei Y, Liu Y, Fu J, Wu T, Chen W, Jiang S, Lv M, Zhang J. To establish a model for the prediction of initial standard and maintenance doses of warfarin for the Han Chinese population based on gene polymorphism: a multicenter study. Eur J Clin Pharmacol 2022;78(1):43-51.
Figures