3623
Time-efficient Relaxation-Incorporated IVIM Diffusion Imaging1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States, 3Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Keywords: Data Acquisition, Diffusion/other diffusion imaging techniques
Overestimation of perfusion volume fraction has been reported in conventional IVIM imaging. In a model known as extended T2-IVIM, compartmentalized relaxation times are incorporated to improve quantification of perfusion volume fraction, which requires acquisition of additional images at different TEs. A main challenge is that the scan time is lengthened, decreasing the efficiency while increasing vulnerability to motion. We herein introduce a novel sequence for time-efficient, relaxation-incorporated (TERI) IVIM imaging to address the aforementioned issues. TERI IVIM imaging uses multiple EPI readouts at different TEs in a single shot. The proposed technique has been demonstrated in the human brain.Introduction
The intra-voxel incoherent motion (IVIM) diffusion model, originally proposed by Le Bihan et al. 1, has attracted renewed attention. In this model, the diffusion-weighted MR signal at a given echo time (TE) is attenuated by pseudo-diffusion (or perfusion) and true-diffusion: $$$ S\left ( b,TE \right )/S_{0}= exp\left ( -TE/T2 \right )\times \left [ fexp\left ( -bD^{^{\ast }} \right )+\left ( 1-f \right )exp\left ( -bD \right ) \right ]$$$. In this two-compartmental model, f is the perfusion volume fraction, D* and D are the pseudo- and true-diffusion coefficients, respectively, and T2 is the transverse relaxation time which is typically assumed to be the same in both compartments. However, blood associated with the perfusion compartment is known to have a longer T2 than tissues, leading to an overestimated perfusion volume fraction f 2–5. In a model known as extended T2-IVIM 3, the compartmentalized relaxation times can be accounted for by acquiring additional images in multiple scans, each with a distinctive TE. This approach, however, leads to a substantial increase in the total scan times and hence vulnerability to motion. We herein report a time-efficient technique that acquires diffusion-weighted signals at multiple TEs following a single excitation. We call this technique time-efficient, relaxation-incorporated (TERI) IVIM imaging. The signals from TERI-IVIM are analyzed using an extended T2-IVIM model to simultaneously characterize perfusion, diffusion, relaxation, and volume fraction.Methods
Pulse sequence design: The sequence for TERI-IVIM imaging was based on a spin-echo diffusion-weighted EPI sequence in which multiple (e.g., 3) EPI readout echo-trains were placed after a Stejskal-Tanner diffusion preparation module (Figure 1). Each EPI readout echo-train corresponded to a distinct effective TE, where the effective TE is defined as the TE when the k-space center is sampled. The first echo-train coincided with the nominal TE (TE0). Following the first readout echo-train, a180° RF refocusing pulse was applied to refocus the signal for the second echo-train acquisition. This process was repeated until the last echo-train was acquired. To reduce the length of each echo-train without compromising the spatial resolution, thereby enabling acquisition of multiple echo-trains, GRAPPA was employed with a two-fold acceleration.Experiments: The TERI-IVIM imaging sequence was implemented on a GE MR750 3T scanner. Using this sequence, axial brain images were acquired with a 32‐channel head coil on healthy subjects. The acquisition parameters were: FOV=192×192 mm2, imaging matrix=64×64, number of slices=6, slice thickness=5 mm, slice spacing=1 mm, TR=4000 ms, TEs=50.5/80.0/109.5 ms, number of echo-trains=3, echo-train length for each train=32, b=0/20/50/100/150/200/500 s/mm2, NEX=6/6/6/6/8/10/16, and the scan time=11 min and 40 s.
Data analysis: Diffusion-weighted images with different b-values acquired at different TEs were separately reconstructed. The voxel-level signal in these images can be described by 3:$$S\left ( b,TE \right )/S_{0}=fexp\left ( -TE/T2_{p} \right )exp\left ( -bD^{^{\ast }} \right )+\left ( 1-f \right )exp\left ( -TE/T2_{d} \right )exp\left ( -bD \right )$$where S0 is the signal intensity with b-value = 0 s/mm2 and TE = 0 ms, and T2p and T2d are the T2 values of the perfusion and true-diffusion compartments, respectively. In comparison with the conventional IVIM model, the model above can simultaneously determine compartmentalized perfusion, diffusion, and T2 relaxation parameters. The model was used to fit to all 21 images (7 b-values × 3 TEs) voxel-by-voxel using a non-linear least-squares fitting algorithm in MATLAB 2021a (MathWorks, Inc.). Fitting was repeated 100 times with randomized initial parameters to avoid local minima. For comparison, a conventional IVIM model was also fitted to the diffusion-weighted images acquired at TE0. Regions of interest (ROIs) were drawn only in the white matter to minimize the partial-volume effects from cerebrospinal fluid (Figure 2). After obtaining the voxel-wise parameter maps, the mean value of perfusion volume fraction within the ROIs was compared between TERI-IVIM and conventional IVIM imaging.
Results
TERI-IVIM imaging enabled a three-fold acceleration without compromising the spatial resolution when compared with conventional separate scans with different TEs. Using the TERI-IVIM sequence, two-dimensional diffusion-weighted brain images (Figure 2) from a representative healthy subject with seven b-values and three TEs were obtained within 12 minutes. TERI-IVIM imaging provided information not only on perfusion (D*) and diffusion (D), but also on compartmentalized T2 values, thereby removing the T2/TE bias in perfusion fraction quantification. Figure 3 shows that the model fits the measured data well with respect to both b-value and TE [RSE (relative squared error) < 0.01]. The resultant parameter maps are shown in Figures 4. Quantitative measurement of perfusion volume fraction in the white matter revealed an approximately 30.4% reduction in TERI-IVIM imaging (f = 0.064) compared with conventional IVIM imaging (f = 0.092). The perfusion volume fraction maps of a representative slice obtained with the two techniques are shown in Figure 5, together with their difference map.Discussion and Conclusion
We have demonstrated a novel technique, TERI-IVIM imaging, that can be used to acquire multiple diffusion-weighted images at different TEs in a single shot. The three-fold improvement in time efficiency has enabled quantification of perfusion, diffusion, relaxation, and volume fraction in the human brain in less than 12 minutes. Our observation that TERI-IVIM produced a lower perfusion volume fraction than conventional IVIM imaging demonstrates the ability to reduce overestimation. The TERI-IVIM sequence is expected to stimulate ongoing research to expand and refine the IVIM model for diffusion imaging studies.Acknowledgements
This work was supported in part by the National Institutes of Health (5R01EB026716-01 and 1S10RR028898-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Muge Karaman, Alessandro Scotti, and Kezhou Wang for helpful discussions.References
1. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497-505.
2. Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med. 2010;64(6):1580-1585.
3. Jerome NP, D’Arcy JA, Feiweier T, et al. Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys Med Biol. 2016;61(24):N667-N680.
4. Feng Z, Min X, Wang L, et al. Effects of Echo Time on IVIM Quantification of the Normal Prostate. Sci Rep. 2018;8(1):2572.
5. Egnell L, Jerome NP, Andreassen MMS, Bathen TF, Goa PE. Effects of echo time on IVIM quantifications of locally advanced breast cancer in clinical diffusion-weighted MRI at 3 T. NMR Biomed. 2022;35(5):4654.
Figures
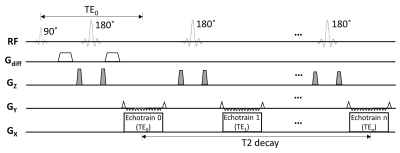
Figure 1: A diagram of the pulse sequence for TERI-IVIM imaging. Multiple EPI echo-trains were incorporated into the sequence, with each echo-train corresponding to a distinctive TE (TE0, TE1, …, TEn). Between any two adjacent echo-trains, a 180° RF refocusing pulse was applied to refocus the signal for the subsequent echo-train acquisition. The refocusing pulses were typically accompanied by a crusher gradient pair (shaded in gray) with varying amplitudes to eliminate unwanted stimulated echo signals. For simplicity, the slice-selection gradients on the Gz axis are omitted.
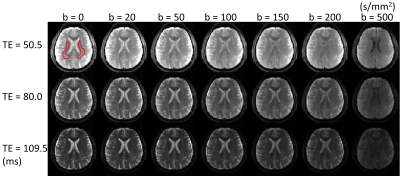
Figure 2: A set of TERI-IVIM diffusion-weighted brain images with seven b-values and three TEs from a representative healthy subject (male, 26 years of age), resulting in 21 images. Regions of interest (ROI) were drawn in the white matter on the T2-weighted image (b = 0 s/mm2, TE = 50.5 ms), as indicated by the red contours.

Figure 3: Data fitting in TERI-IVIM imaging (A) and conventional IVIM imaging (B) for a representative voxel in the brain. Compared with conventional IVIM imaging, TERI-IVIM imaging accounts for the compartmentalized T2 relaxation. Both models fit the measured values (red stars) very well (RSE < 0.01).
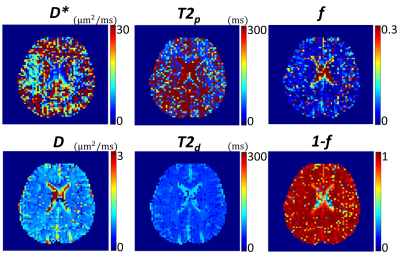
Figure 4: Parameter maps of D*, D, T2p, T2d, f, and (1-f) calculated from TERI-IVIM imaging with 21 diffusion-weighted images acquired using 7 b-values and 3 TEs.
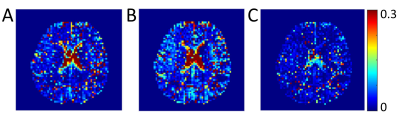
Figure 5: Parameter maps of perfusion volume fraction (f) calculated from TERI-IVIM imaging (A) and conventional IVIM imaging (B). The differences of the two maps (f IVIM - f TERI-IVIM) are shown in (C).