3622
Segmented thick-slab 3D DWI with first and second order motion-compensated diffusion gradients1Radiology, Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Medical Radiation Sciences, Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 3Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 4Radiology, Sahlgrenska University Hospital, Gothenburg, Sweden, 5Radiology, Brigham and women's hospital, Boston, MA, United States
Synopsis
Keywords: Data Acquisition, Diffusion/other diffusion imaging techniques, Motion correction, 3D imaging
Routine clinical diffusion imaging is generally performed with 2D echo planar sequences. A single thick-slab 3D approach could offer higher signal-to-noise ratio and better slice resolution, but has not been adopted due to the difficulty to avoid motion-induced phase errors that interfere with multi-shot spatial encoding. A new approach to enable 3D DWI is introduced here: rather than relying on navigator echoes for phase correction, first and second order motion-compensated diffusion encoding gradients are used to minimize phase variations at the source.Introduction
Diffusion-weighted imaging (DWI) predominantly relies on a single-shot 2D data acquisition scheme. A 3D acquisition would allow for superior signal-to-noise ratio (SNR)1 but cannot be performed in a single readout and, therefore, requires segmentation. With a multi-shot readout approach, the shot-to-shot phase variations that arise from bulk tissue movement in the presence of motion probing gradients severely interfere with spatial encoding and lead to pronounced ghosting and blurring artifacts. One approach to overcome this limitation is to perform the 3D acquisition for a thin slab2–5. The thickness is limited by the assumption that for thin slabs motion-related through-slice phase variation is minimal and that hence in-plane 2D phase navigator correction suffices. A 3D navigator is not practical since inherent low sampling bandwidth would result in tremendous distortions and/or inadequate k-space coverage. Another approach is to repetitively employ a diffusion preparation sequence to generate diffusion-weighted longitudinal magnetization followed by an independent excitation and segmented readout6. This preparation approach sacrifices half the signal. We propose an alternative approach based on motion-compensated diffusion encoding gradients, with the goal to minimize motion-related shot-to-shot phase variations so that navigation could be simplified or completely omitted. In the early phase of development of robust diffusion imaging sequences, a 2D-segmented approach with velocity compensation using bi-polar diffusion gradients has been reported7. Unfortunately, this approach is associated with pronounced sensitivity for higher order motion terms. Time-efficient velocity and acceleration-compensated diffusion encoding based on trapezoid waveforms with low sensitivity for higher order motion terms have been introduced for single-shot diffusion imaging in the heart8.Methods
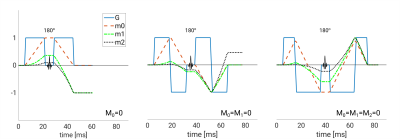
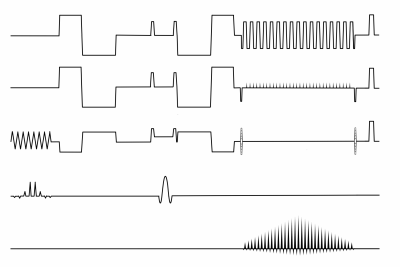
Two healthy volunteers were scanned on a 3 Tesla scanner (Premier, GE Healthcare, Milwaukee WI, USA) with a 48-channel head coil (Gmax = 80mT/m, slew rate = 200 mT/m/ms). Three scans with moment nulling of zeroth order (m0=0), zeroth and first order (m0=m1=0), and zeroth, first, and second order (m0=m1=m2=0), respectively, with a single thick slab excitation of 10 cm, were performed (Fig. 1). The 3D sequence employs two phase encoding directions, whereby the second phase encoding pulse is incremented with each shot followed by an in-plane echo-planar readout orthogonal to the second phase encode direction (Fig. 2). A navigator was not employed.Sequence parameters common to all scans were TR: 1000 ms; x,y,z resolution: 1.5x1.5x1,5 mm3; matrix x,y,z: 144x144x80; FOVx,y,z: 22x22x12 cm, three orthogonal diffusion directions and b-values of 0 and 1000 s/mm2; in-plane multi-coil acceleration: R=2. Total scan time for each scan was 5:21 min with echo times of 82 ms (m0=0), 118 ms (m0=m1=0) and 123 ms (m0=m1=m2=0). All data was reconstructed using MatLab (2021b, Mathworks, Natick, MA, USA).
Results
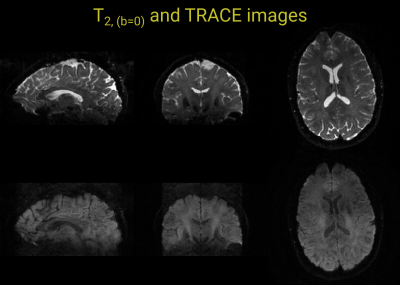
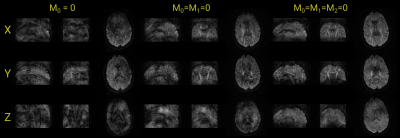
Coronally, sagitally, and axially reformatted images of the 3D T2-weighted (b0) and trace DWI data obtained with first and second order motion compensation are shown in Figure 3. Some residual ghosting artifacts can be observed along the superior-inferior direction on the sagittal and coronal images. On the axial image, elevated signal in the ventricles and some signal loss in the frontal half of the brain is evident. In Fig. 4, coronally, sagitally, and axially reformatted images for each of the applied diffusion encoding directions are shown for scans with m0=0, m0=m1=0, and m0=m1=m2=0, respectively. The most severe artifacts arise with the configuration that uses no motion compensation and the least artifacts result with the scan that uses both first and second order moment nulled diffusion encoding gradients. Moreover, there was clear evidence of encoding-strength dependent banding artifacts, that appeared along both edges of the selected slab, predominantly during the application of diffusion encoding along the y-direction. Phantom tests on another system with lower gradient performance, seemed to indicate that the magnitude and association with gradient direction is not consistent among systems.Discussion and conclusion
With first and second order motion-compensated diffusion encoding gradients, multi-shot images of remarkable quality, high spatial resolution and high SNR can be obtained without navigator-based phase correction. The results indicate that this gradient scheme successfully can minimize the majority of phase errors in 3D-DW thick slab imaging. Extensive verification with phantoms seemed to indicate that banding artifacts and some of the ghosting can be attributed to eddy currents. With clinical 2D DWI a slice thickness below 2 mm is not practical. A 3D-DWI acquisition overcomes this limitation by permitting thinner slices suitable for multi-planar reformation of the image data. This would be beneficial for the investigation of smaller lesions, which otherwise exhibit low contrast due to partial volume effects. Motion compensation implies a longer echo time. Remarkable is that first and second order motion compensation compared with first order compensation only, can be attained with a very minor 4% increase in echo time. More advanced gradient encoding waveforms allow for even further reduction9,10. A thick slab excitation requires a long repetition time in order to avoid T1 weighting. The addition of a second phase encoding direction, however, opens up the possibility for additional acceleration methods, such as multi-coil acceleration or compressed sensing, which, moreover, can be applied in combination.In conclusion, these preliminary results indicate that the use of motion compensated diffusion gradients may be a viable avenue to perform single slab 3D-DWI with full brain coverage.
Acknowledgements
Barncancerfonden
The Swedish state under an agreement between the Swedish government and the country councils (ALFGBG-932648)
The Swedish Research Council (Vetenskapsrådet)
The Swedish Cancer Society (Cancerfonden)
References
1. Engström M, Mårtensson M, Avventi E, et al. On the signal-to-noise ratio efficiency and slab-banding artifacts in three-dimensional multislab diffusion-weighted echo-planar imaging. Magn. Reson. Med. 2015;73(2):718–725.
2. Van AT, Hernando D, Sutton BP. Motion-induced phase error estimation and correction in 3D diffusion tensor imaging. IEEE Trans. Med. Imaging 2011;30(11):1933–1940.
3. Engström M, Skare S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magn. Reson. Med. 2013;70(6):1507–1514.
4. Frost R, Miller KL, Tijssen RHN, et al. 3D multi-slab diffusion-weighted readout-segmented EPI with real-time cardiac-reordered k-space acquisition. Magn. Reson. Med. 2014;72(6):1565–1579.
5. Chang HC, Sundman M, Petit L, et al. Human brain diffusion tensor imaging at submillimeter isotropic resolution on a 3Tesla clinical MRI scanner. Neuroimage 2015;118:667–675.
6. Xie Y, Yu W, Fan Z, et al. High resolution 3D diffusion cardiovascular magnetic resonance of carotid vessel wall to detect lipid core without contrast media. J. Cardiovasc. Magn. Reson. 2014;16(1):1–10.
7. Brockstedt S, Thomsen C, Wirestam R, et al. Use of an enhanced gradient system for diffusion MR imaging with motion-artifact reduction. Acta radiol. 1995;36(4–6):662–670.
8. Stoeck CT, Von Deuster C, GeneT M, et al. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn. Reson. Med. 2016;75(4):1669–1676.
9. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion-weighted MRI. Magn. Reson. Med. 2017;77(2):717–729.
10. Peña-Nogales Ó, Zhang Y, Wang X, et al. Optimized Diffusion-Weighting Gradient Waveform Design (ODGD) formulation for motion compensation and concomitant gradient nulling. Magn. Reson. Med. 2019;81(2):989–1003.
Figures