3621
High-resolution Diffusion Tensor Imaging with Deep Learning Reconstruction: Preliminary Results in Sub-cortical Fiber Tracking
Zhangxuan Hu1, Xiaocheng Wei1, Jie Lu2, and Bing Wu1
1GE Healthcare, Beijing, China, 2Department of Radiology and Nuclear Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
1GE Healthcare, Beijing, China, 2Department of Radiology and Nuclear Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is a well-established tool for providing insights into brain structural connectivity and detecting brain microstructure. High spatial resolution diffusion MRI can provide improved resolvability of fibers with high-curvature (u-fibers). Segmented k-space methods such as Multiplexed sensitivity-encoding (MUSE) are often used to achieve high resolution diffusion images, however the shortcomings, such as prolonged scan time and low signal-noise-ratio (SNR), still exist. In this study, we aim to further improve the image quality of high-resolution diffusion images acquired with MUSE by combing with a deep learning based reconstruction method and thus to improve the sub-cortical fiber tracking accuracy.INTRODUCTION
Diffusion tensor imaging (DTI) is a well-established tool for providing insights into brain structural connectivity and detecting brain microstructure. It has been reported that compared with high angular resolution diffusion MRI techniques 1, high spatial resolution diffusion MRI can provide improved resolvability of fibers with high-curvature 2, such as sub-cortical short association fibers connecting adjacent gyri (u-fibers) 3. However, diffusion images suffer from many artifacts, such as distortion, Gibbs ringing, and low signal-noise-ratio (SNR), which might be more severe for high resolution acquisitions. Multiplexed sensitivity-encoding (MUSE) 4 technique can help to mitigate these challenges and achieve higher resolution. Denoising methods based on PCA can improve the SNR, but the performance is dependent on the reconstruction parameters. Moreover, Gibbs ringing artifacts are still present and may affect the quantitative measurements from diffusion images. Recently, a deep learning based reconstruction method was proposed (DLRecon) 5, which can denoise images and correct for Gibbs ringing artifacts simultaneously. In this study, we aim to combine this method with high spatial resolution diffusion imaging to improve the image quality, and thus to improve the tracking accuracy of sub-cortical fibers.METHODS
MRI data were acquired using a 3.0-Tesla MR system (SIGNA Premier, GE Medical Systems, Milwaukee, WI) using a 48-channel head coil. MUSE was used to acquire whole brain DTI data with parameters as followed: resolution = 1×1×1mm3, b-values = 0, 800 s/mm2, number of directions = 15, number of shots = 4, TR = 22.2s, TE = 64ms, FOV = 256×256×150 mm3, flip angle = 90°. The scan time was about 24 minutes. The DTI images were reconstructed with conventional MUSE and MUSE combined with DLRecon. For comparison, a MP-PCA based method 6 implemented in MRtrix3 (https://www.mrtrix.org) 7 was also applied to the original images reconstructed with conventional MUSE to remove the noise. The reconstructed DTI images were further preprocessed for eddy current correction and distortion correction with FSL (https://fsl/fmrib.ox.ac.uk/fsl/) 8. MRtrix3 was then used to generate diffusion tensors and finally derive deterministic streamline fiber tracts. By limiting the fiber length to >5 mm and <30 mm, sub-cortical u-fibers can be delineated specifically.RESULTS and DISCUSSION
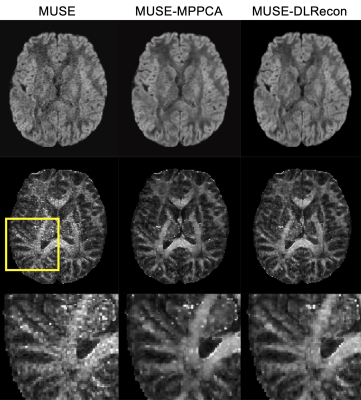
Fig. 1 showed representative diffusion-weighted images reconstructed using the original MUSE, MUSE with MP-PCA denoising and MUSE with DLRecon, as well as corresponding FA maps. Compared to the original MUSE, both DLRecon and MP-PCA improved the SNR. However, compared with MP-PCA, DLRecon further removed Gibbs ringing artifacts and can reduce the quantification errors in the derived FA maps. The FA map from MP-PCA showed much more singular values (as shown in the zoomed-in area in the third row in Fig. 1), especially on the boundaries between different kinds of tissues, which may affect the sub-cortical fiber tracking. Fig. 2 showed the u-fibers generated with MRtrix3 from the DTI images reconstructed with different methods. As shown in the zoomed-in area, with original MUSE, the u-fibers cannot be correctly tracked. Compared with MP-PCA, the fibers generated from DLRecon were more continuous and intact, which demonstrate the advantages of DLRecon in improving the accuracy of sub-cortical fiber tracking.CONCLUSION
Both MP-PCA and DLRecon can denoise high-resolution diffusion images effectively. However, DLRecon enables corrections of Gibbs ringing artifacts, which can help with improving FA measurements and thus to improve the tracking quality of sub-cortical fibers.Acknowledgements
No acknowledgement found.References
1. Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2002;48(4):577-582.2. Bruce I, Petty C, Chang HC, Chen NK, Song AW. The impact of High-Q and High-K on complex fiber structures in the human brain. 2017; Honolulu, ISMRM.
3. Schüz A, Miller R. Cortical areas: unity and diversity: CRC press; 2002.
4. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage 2013;72:41-47.
5. Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:200806559 2020.
6. Cordero-Grande L, Christiaens D, Hutter J, Price AN, Hajnal JV. Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage 2019;200:391-404.
7. Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. International journal of imaging systems and technology 2012;22(1):53-66.
8. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage 2012;62(2):782-790.
DOI: https://doi.org/10.58530/2023/3621