3614
Feasibility of diffusion imaging using SMS-spiral acquisition with corrections on gradient waveform and field inhomogeneities1Techna Institute, University Health Network, Toronto, ON, Canada, 2University of British Columbia, Vancouver, BC, Canada, 3Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Pulse Sequence Design, Diffusion/other diffusion imaging techniques, Spiral Imaging, field inhomogeneity correction, gradient correction
We propose a short-TE signal-to-noise ratio (SNR) enhanced diffusion imaging method using simultaneous multi-slice (SMS) accelerated spiral acquisition. The correction of field inhomogeneity and gradient waveforms are introduced in the reconstruction without any assistance of external hardware (e.g. field camera). Results showing the feasibility of this method on both phantom and human subjects, and the corrections of B0 and gradient waveforms are essential to improve the image quality.Introduction
Diffusion imaging has popular clinical and research applications, including but not limited to brain development, stroke, and demylination. The traditional EPI-based method is limited by the signal-to-noise ratio (SNR) and T2 blurring due to the long EPI readout train. The spiral readout has a much shorter echo time (TE) over EPI and has been proven with a higher SNR efficiency [1]. Previous studies have shown that spiral acquisition is able to achieve a substantial reduction of echo time and thus improve SNR for diffusion imaging [2]. In this study, we propose using a simultaneous multi-slice (SMS) technique to accelerate the acquisition of spiral diffusion images with field inhomogeneity and gradient waveforms corrected without any assistance of external hardware (e.g. field camera).Methods
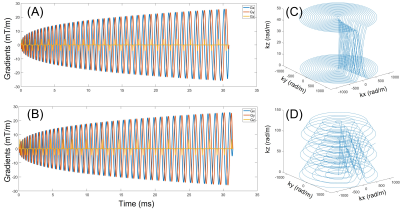
Trajectory designing: Figure 1 is a demonstration of the spiral readouts for SMS 2 and SMS 3, together with their k-space trajectories. We adopted the T-Hex shaped gradient blips [3] along the slice-selection direction for encoding purposes, as we are using a 3D k-space approach [4] to reconstruct all SMS slices. The purpose of the T-Hex blips is to reach a near-uniform k-space density that leads to a near-optimal SNR efficiency and a benign T2* effect.Acquisition: The spiral diffusion sequence was in-house developed, and all data were acquired on a Siemens 3T Prisma scanner (Erlangen, Germany). Spiral diffusion images with SMS acceleration factors of 2 were acquired on a phantom and a healthy subject (with approval of REB), as well as the corresponding non-SMS spiral images. The common scanning parameters are TR = 8000 ms, TE = 40 ms, in-plane FOV 220 mm, in-plane resolution 2.6 mm, slice thickness 2 mm, b-values 0 and 700 s/mm2, and 60 slices acquired. An additional dual-echo GRE dataset (TE 4.9/7.4 ms) was acquired to calculate B0 and coil sensitivity maps.
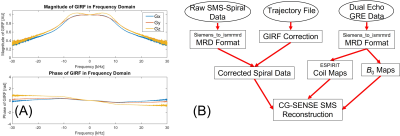
Gradient Characterization: A phantom-based method was used to measure and calculate the gradient impulse response function (GIRF) with a modified GRE sequence including a gradient blip before readouts with a slew rate of 180 T/m/s [5]. There were 18 blip amplitudes (from 9 to 39.6 mT/m) included in the measurement of GIRF [6]. The measured GIRFs on three gradient axis that were subsequently used for k-space trajectory correction are shown in Figure 2A.
Image Reconstruction: A phase correction was applied to the raw k-space signal to compensate for the shift from the iso-center of the magnet [4]. All reconstructions of spiral images and the GIRF corrections over the spiral readouts were implemented in Julia language, using the open-source library MRIReco.jl [7] and ISMRM Raw Data [8] under a reconstruction framework we previously proposed in [9] and is shown in Figure 2B. A nonlinear conjugate gradient solver (CG-SENSE) including the B0 term was used for reconstruction.
Results
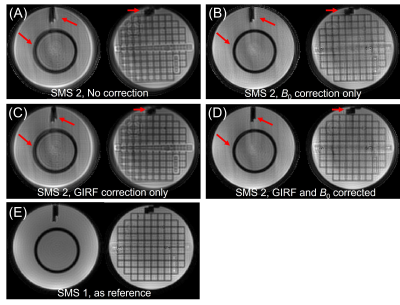
Figure 3 illustrates the necessity of both field inhomogeneity correction and gradient waveform compensations. Two slices from one slice group of the SMS 2 spiral phantom images (b = 0) are used for this demonstration with the non-SMS spiral images shown as the reference. The result demonstrates that the B0 and gradient corrections are both essential for SMS spiral acquisition: B0 correction recovers blurring and distortions on the area with high field inhomogeneity (red arrows), while GIRF gradient correction compensates the object position changes (e.g. rotation) due to the deviation between nominal and actual k-space trajectories. After these corrections, the image quality becomes nearly equivalent to the non-SMS cases.Figure 4 shows the effect of B0 correction on one slice group of the human subject with SMS 2 under b-value 0 and 700 s/mm2. All sub-images in this figure went through GIRF correction. B0 correction helps recover the blurring and artifacts in the region with significant field inhomogeneities.
Discussion and Conclusion
This study demonstrated the feasibility of SMS acceleration over spiral diffusion-weighted images using a 3D framework for reconstruction. The minimum TR for SMS 2 acquisition was 4000 ms for 60 slices; the purpose of setting it as 8000 ms was to match the contrast of non-SMS cases. On the other hand, the spiral readout reduced TE dramatically: a protocol reaching a similar resolution and b-value under EPI-based DWI acquisition (with echo train length reduced through parallel imaging and partial Fourier) still need a TE > 70ms, while our acquisition only needs 40ms.Future investigations of this work include: (1) A higher SMS factor would be desirable to further reduce the scanning time; (2) The diffusion weighting gradients may need to be included in GIRF calculation together with the spiral gradients to compensate for its strong eddy current with long time coefficients; (3) A multi-interleaved spiral acquisition would give a higher resolution;(4) The validity of this spiral SMS diffusion imaging needs to be verified under multiple diffusion models such as DTI, NODDI, etc.
In a conclusion, we demonstrated the feasibility of SMS spiral acquisition and reconstruction for diffusion imaging. The field inhomogeneity and gradient corrections are essential to improve the image qualities, noting that no additional hardware is needed for corrections during image reconstruction.
Acknowledgements
The authors thank Dr. Johanna Vanesjo (Norwegian University of Science and Technology), Dr. Maria Engel (Cardiff University), and Dr. Gerald Moran (Siemens Healthineer) for their advice on this work.References
[1] Yoojin Lee, Bertram J. Wilm, David O. Brunner, Simon Gross, Thomas Schmid, Zoltan Nagy, Klaas P. Pruessmann. On the signal-to-noise ratio benefit of spiral acquisition in diffusion MRI. Magnetic Resonance in Medicine 2021;85: 1924 – 1937. https://doi.org/10.1002/mrm.2855
[2] Bertram Jakob Wilm, Franciszek Hennel, Manuela Barbara Roesler, Markus Weiger, Klaas Paul Pruessmann. Minimizing the echo time in diffusion imaging using spiral readouts and a head gradient system. Magnetic Resonance in Medicine 2020;84: 3117–3127. https://doi.org/10.1002/mrm.28346
[3] Maria Engel, Lars Kasper, Bertram Wilm, Benjamin Dietrich, Laetitia Vionnet, Franciszek Hennel, Jonas Reber, Klaas P. Pruessmann. T-Hex: Tilted hexagonal grids for rapid 3D imaging. Magnetic Resonance in Medicine 2021;85:2507–2523. https://doi.org/10.1002/mrm.28600
[4] Benjamin Zahneisen, Benedikt A. Poser, Thomas Ernst, and V. Andrew Stenger. Three-Dimensional Fourier Encoding of Simultaneously Excited Slices: Generalized Acquisition and Reconstruction Framework. Magnetic Resonance in Medicine 2014;71:2071–2081. https://doi.org/10.1002/mrm.24875
[5] Zhe Wu, Alexander Jaffray, Johanna Vannesjo, Kamil Uludag, and Lars Kasper. MR Gradient System Long-Term Stability Investigation and Protocol Optimization for Quality Control using Gradient Impulse Response Function (GIRF). Proceeding of ISMRMR 2022, pp 0641.
[6] Vannesjo, S. J., Haeberlin, M., Kasper, L., Pavan, M., Wilm, B. J., Barmet, C., & Pruessmann, K. P. (2013). Gradient system characterization by impulse response measurements with a dynamic field camera. Magnetic Resonance in Medicine, 69(2), 583–593. https://doi.org/10.1002/mrm.24263
[7] Tobias Knopp, Mirco Grosser. MRIReco.jl: An MRI reconstruction framework written in Julia. Magnetic Resonance in Medicine 2021;86:1633–1646. https://doi.org/10.1002/mrm.28792
[8] Souheil J. Inati, Joseph D. Naegele, Nicholas R. Zwart, Vinai Roopchansingh, Martin J. Lizak, David C. Hansen, Chia-Ying Liu, David Atkinson, Peter Kellman, Sebastian Kozerke, Hui Xue, Adrienne E. Campbell-Washburn, Thomas S. Sørensen, Michael S. Hansen. ISMRM Raw data format: A proposed standard for MRI raw datasets. Magnetic Resonance in Medicine 2017;77:411–421. https://doi.org/10.1002/mrm.26089
[9] Alexander Jaffray, Zhe Wu, Kamil Uludag, and Lars Kasper. Open-source model-based reconstruction in Julia: A pipeline for spiral diffusion imaging. Proceeding of ISMRMR 2022, pp 2435.
Figures