3613
Cardiac DTI with spiral readouts with third order k-space correction but without inner volume excitation1Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom, 2Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 3Siemens Healthcare Ltd, Camberly, United Kingdom, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Medical Radiation Physics, Clinical Sciences Lund, Lund University, Lund, Sweden, 6Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Pulse Sequence Design, Diffusion Tensor Imaging, Spiral
Using spiral trajectories instead of EPI can reduce the echo time in diffusion weighted MRI. The feasibility of spiral trajectories for cardiac DTI has recently been demonstrated, but the larger object size (i.e torso vs. for example skull) may require an inner volume excitation to keep the readout at an useable duration. Here we examine the use of an undersampled spiral with SENSE reconstruction and standard slice selective pulses. We show that MD and FA derived from the slice selective and inner volume excitation yield comparable values with the MD being higher than the one measured with EPI.
Introduction
Spiral readouts have been shown to reduce the echo time (TE) and increase signal to noise ratio (SNR) in diffusion tensor imaging (DTI)1–3. The adoption of spiral readouts is growing, in part due to the development of an expanded signal model for the image reconstruction4,5 which incorporates an extension of the k-space to higher spatial orders and other confounding variables like B0 inhomogeneities. Another reason is the improvement of techniques to measure the true k-space trajectory5–7.While the development of spiral readouts for DTI has primarily been focused on brain DTI, the use of spirals has also been recently demonstrated for cardiac DTI using STEAM8,9 and spin echo (SE)10 based sequences. These methods used inner volume excitation, either with 3 orthogonal 90° rf pulses (STEAM) or a 2D 90° rf pulse and a slice selective 180° rf pulse (SE). This adds an extra layer of complexity to the imaging as these rf-pulses are not necessarily readily available on every MRI scanner.
Furthermore, the published cardiac spiral DTI studies either used no8,9 or only first order10 trajectory correction, measuered in a phantom. All the acquisitions were carried out during breath-hold.
Here we present free breathing cardiac DTI with spiral readouts and third order k-space correction for image reconstruction measured with a field camera. We examine the benefit of an inner volume excitation, which might not be readily available on every MRI scanner.
Methods
We acquired cardiac dMRI on a 3T MRI (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany) with a maximal gradient amplitude of 80 mT/m. Data were acquired in 2 healthy volunteers who provided written consent. The imaging protocols consisted of:(i) two GRE cine sequences with two TEs (3.69 and 6.15 ms) for B0-map estimation (resolution 1.73$$$\times$$$1.73 mm2), Grappa: 2
(ii) a short EPI scout with three directions and b = 0.45 ms/µm2 for coil sensitivity estimation,
(iii) prototype EPI SE diffusion with 2d excitation (Zoom-IT) (Fig. 1a),
(iv) spiral SE diffusion with 2D excitation (Zoom-IT) (Fig. 1b),
(v) spiral SE diffusion with slice selective excitation (Fig. 1c).
All diffusion imaging was performed in free breathing with a nominal resolution of 2.3$$$\times$$$2.3 mm2 and a slice thickness of 8 mm (3 slices in total). The spirals were designed with an optimal gradient design algorithm11 for a field of view of 320$$$\times$$$320 mm2 and an undersampling of 2 which led to a readout duration of 30 ms. The EPI was acquired with partial Fourier of 7/8. TR per slice was 3RR-intervals, TE was set to the minimal possible value (77 ms for EPI, 75 ms for spiral with Zoom-IT, 56 ms for spiral with slice selective excitation). Saturation bands were placed to suppress the signal from outside the volume of interest, 2 in phase encoding direction for the EPI, 4-6 around the heart for the spiral. The EPI for coil sensitivities used the same settings as the main diffusion EPI except for no partial Fourier and a longer TE (84 ms).
The Zoom-IT pulse was limiting the excitation in slice and (nominal) phase direction, while the 180 was always in slice direction, leading only to partial inner volume excitation.
The b-values were 0.1 (3 directions) and 0.45 (30 directions) ms/µm2 with 6 repetitions. For the diffusion encoding, second order motion compensated gradients were used.
The trajectory of the spiral sequences was measured with a field camera (Skope Magnetic Technologies, Zurich, Switzerland). Field camera and MRI raw data were aligned using an iterative method12. The images were reconstructed with an expanded signal model in Matlab (The MathWorks Inc, Natick, Natick, Massachusetts) with lsqr.
Results
Figure 2 shows the improvements in the reconstruction by including the measured trajectory and B0-maps in the signal model. It also demonstrates the advantage of an inner volume excitation with the Zoom-IT pulse. The spiral images are still more blurred than the EPI.The averaged diffusion (Figure 3) weighted images show some artefacts which are more prevalent in the slice selective acquisition than in the Zoom-IT one.
The fractional anisotropy (FA) and mean diffusivity (MD) maps (Fig .4) show similar results between the two spiral acquisitions. The FA values of the spiral acquisitions are comparable to the EPI, the MD values are slightly higher (Table 1). The increase in the MD was not visible in a phantom experiment with different concentrations of PVP solution (data not shown).
Discussion
Here we show initial results for free breathing cardiac DTI without inner volume excitationThe higher MD compared to EPI was also previously reported in cardiac DTI with spiral readouts8–10. A possible explanation for this is the higher SNR in spiral images even at similar TE to the EPI1 (not yet checked in this work) which would reduce the underestimation of MD due to the low SNR of the EPI13. Here we used a third order expansion of the measured k-space, while previous work only corrected for linear terms10. Future work will examine the improvement achieved by this correction more in detail.
Conclusion
We demonstrate the feasibility of cardiac DTI without inner volume excitation. The diffusion parameters are comparable to the ones with inner volume excitation in one in-plane imaging dimension.Acknowledgements
We thank Siemens Healthcare for the pulse sequence development environment. This work was supported by the Wellcome Trust (219536/Z/19/Z) and the British Heart Foundation (PG/19/1/34076).References
1. Lee Y, Wilm BJ, Brunner DO, et al. On the signal-to-noise ratio benefit of spiral acquisition in diffusion MRI. Magn Reson Med 2021; 85:1924–1937.
2. Wilm BJ, Hennel F, Roesler MB, Weiger M, Pruessmann KP. Minimizing the echo time in diffusion imaging using spiral readouts and a head gradient system. Magn Reson Med 2020; 84:3117–3127.
3. Mueller L, Rudrapatna SU, Tax CMW, Wise RG, Jones DK. Diffusion MRI with b=1000 s/mm2 at TE < 22 ms using single-shot spiral readout and ultra-strong gradients: Implications for microstructure imaging. Proceedings of the 29th Annual Meeting of ISMRM, Montreal, 2019; 0766
4. Wilm BJ, Barmet C, Gross S, et al. Single-shot spiral imaging enabled by an expanded encoding model: Demonstration in diffusion MRI. Magn Reson Med 2017; 77:83–91.
5. Wilm BJ, Nagy Z, Barmet C, et al. Diffusion MRI with concurrent magnetic field monitoring. Magn Reson Med 2015; 74:925–933.
6. Dietrich BE, Brunner DO, Wilm BJ, et al. A field camera for MR sequence monitoring and system analysis. Magn Reson Med 2016; 75:1831–1840.
7. de Zanche N, Barmet C, Nordmeyer-Massner JA, Pruessmann KP. NMR Probes for measuring magnetic fields and field dynamics in MR systems. Magn Reson Med 2008; 60:176–186.
8. Gorodezky M, Scott AD, Ferreira PF, Nielles-Vallespin S, Pennell DJ, Firmin DN. Diffusion tensor cardiovascular magnetic resonance with a spiral trajectory: An in vivo comparison of echo planar and spiral stimulated echo sequences. Magn Reson Med 2018; 80:648–654.
9. Gorodezky M, Ferreira PF, Nielles-Vallespin S, et al. High resolution in-vivo DT-CMR using an interleaved variable density spiral STEAM sequence. Magn Reson Med 2019; 81:1580–1594.
10. Gorkum RJH van, Guenthner C, Koethe A, Stoeck CT, Kozerke S. Characterization and Correction of Diffusion Gradient-Induced Eddy Currents in Second-Order Motion-Compensated Echo-Planar and Spiral Cardiac DTI. Magn Reson Med 2022
11. Lustig M, Kim SJ, Pauly JM. A fast method for designing time-optimal gradient waveforms for arbitrary k-space trajectories. IEEE Trans Med Imaging 2008; 27:866–873.
12. Dubovan PI, Baron CA. Model‐based determination of the synchronization delay between MRI and trajectory data . Magn Reson Med 2022
13. Jones DK, Basser PJ. “Squashing peanuts and smashing pumpkins”: How noise distorts diffusion-weighted MR data. Magn Reson Med 2004; 52:979–993.
Figures
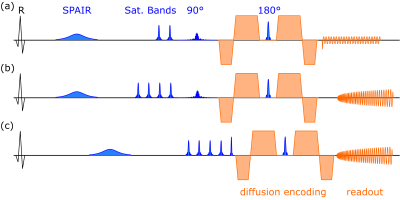
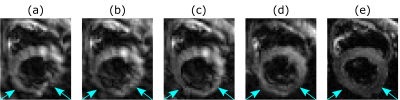
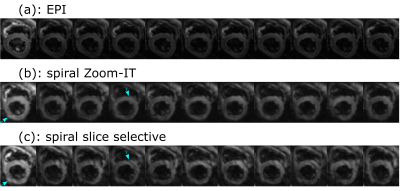
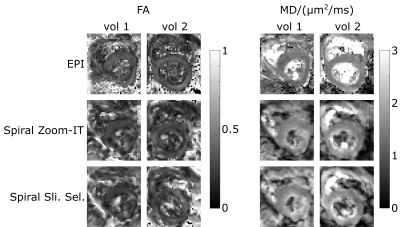
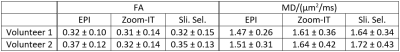
Table 1: Mean ± standard deviation of FA and MD inside a left ventricle mask over the whole ventricle for both volunteers and all three acquisition schemes.