3588
Human brain thalamic nuclei segmentation: compressed-sensing effects on 3D MPRAGE variants
Sebastian Hübner1, Stefano Tambalo1, Tobias Kober2,3,4, and Jorge Jovicich1
1Center for Mind/Brain Sciences, University of Trento, Rovereto (Trento), Italy, 2Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 3Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
1Center for Mind/Brain Sciences, University of Trento, Rovereto (Trento), Italy, 2Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 3Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Keywords: Segmentation, Brain, Thalamus
Accurate segmentation of thalamic nuclei can significantly support clinical decisions, especially in diseases such as Parkinson’s. At the same time, highly accelerated T1w acquisition techniques find wider clinical acceptance as they not only increase radiological efficiency and patient comfort, but also reduce motion artefacts. Here, we study how compressed sensing affects thalamic nuclei volumetry based on MPRAGE or MP2RAGE in healthy subjects with respect to clinical baseline. Segmentation results are promising and agree with expectations: acceleration to about 1 min is possible maintaining overall good image quality, contrast and thalamic segmentations, with acceleration-related biases.Introduction
The thalamus is a collection of grey matter nuclei primarily involved in gating ascending cortical input and modulating cortical activity via transthalamic cortico-cortical pathways1. Thalamic nuclei characterization is of relevance for various patient populations, including Parkinson’s disease2, temporal lobe epilepsy3, and dementia4. One of the technical challenges of mapping thalamic nuclei is the study of patient populations with high levels of head motion. This challenge may be addressed by adopting accelerated MRI strategies in clinical protocols, such as compressed sensing MPRAGE (CS-MPRAGE)5. Several 3T MRI studies demonstrated that with moderate CS accelerations, images show comparable quality to standard protocols6, and that brain tissue volumetric results can be preserved with accelerations up to x6, with biases at higher accelerations7,8. However, the influence of CS acceleration in thalamic nuclei segmentations has not been investigated. In this study, we compared thalamic nuclei segmentations from MP2RAGE (8:52 min) with CS-MP2RAGE (3:40 min) and two differently accelerated CS-MPRAGE scans (2:04 and 1:14 min).Methods
MRI acquisition: Six healthy adults (mean age=25.7, range 23,28 years) underwent 3T MRI (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel head-neck RF receive coil. 3D T1-weighted (T1w) images were acquired with four MPRAGE sequence variants, all with 1mm isotropic voxels, same spatial coverage, prescan normalize on and no image filters: MP2RAGE (TR/TE=5000/2.98ms; α=4°/5°; GRAPPA=3; TA=8:52 min), CS-MP2RAGE (TR/TE=5000/2.88ms; α=4°/5°; samples/TR=195; undersampling factor 4.6; regularization factors=0.0006/0.0004; TA=3:40 min), and CS-MPRAGE (TR/TE/TI=2300/2.88/900ms; α=9°; samples/TR=196; regularization=0.0006) with two different accelerations: i) undersampling factor 3.6; TA=2:04 min, and ii) undersampling factor 6.6; TA=1:14 min. More details on CS reconstructions can be found in reference [6].Thalamic nuclei segmentation: T1w images were processed through the FreeSurfer (v7.1.1) recon-all stream, which performs cortical and subcortical segmentation, then through the thalamic nuclei segmentation tool giving volumetric data for each thalamic nucleus9. Image quality was assessed qualitatively using the denoised MP2RAGE10 as reference.
Statistical analysis: Given volume hemispheric similarities with this small sample size, only right thalamic nuclei are presented. The non-parametric Friedman rank sum test was used to assess sequence effects on the volumes of the whole thalamus and thalamic nuclei. Coefficients of variation (CV) were used as a measure of volume diversity across sequences for the whole thalamus and major thalamic nuclei (PuM: pulvinar medial, VPL: ventral posterolateral, VLp: ventral lateral posterior, MDm: mediodorsal medial magnocellular, VLa: ventral lateral anterior, VA: ventral anterior, MDI: mediodorsal lateral parvocellular, LGN: lateral geniculate).
Results
Fig. 1 shows T1w data in a sample subject for qualitative image quality comparisons across MPRAGE variants. Image contrast is well preserved with acceleration, with expected increased image blurring at higher accelerations. Fig. 2 shows FreeSurfer automated thalamic parcellations across sequences in a sample subject. We observed overall good qualitative agreement of the spatial segmentations between MP2RAGE and both CS-MPRAGE. Fig. 3 shows volumetric findings of the whole thalamus and thalamic nuclei across sequences, respectively. The Friedman test revealed significant sequence effects in nuclei VLp (χ²(3)=12.6, padj<0.01), VLa (χ²(3)=13, padj<0.01), MDm (χ²(3)=12.6, padj<0.01) and MDl (χ²(3)=11, padj<0.05). Fig. 4 shows volume variations (CV) of whole thalamus and thalamic nuclei across sequences, overall below 15%. Table 1 lists the median whole thalamic and thalamic nuclei volumes for the various sequences.Discussion
In agreement with previous 3T studies, we find that acceleration of brain T1w anatomical images to about 1 min acquisition is possible without significant loss of data quality6,7,8,11. Upon visual inspection, thalamic nuclei segmentation quality was in good agreement with the literature9,12, as well as volumetric values9,13,14. The choice of MPRAGE sequence variants introduces variability, but this remains under 15% for the whole thalamus and major thalamic nuclei. We found significant sequence effects in nuclei VLp, VLa, MDm, and MDl. The trend suggests a bias towards larger thalamic nuclei volumes for CS-MP2RAGE. These findings need further evaluation with a larger sample size.Acknowledgements
This work was supported by funding from the Municipality of the City of Rovereto (Trento), Italy, for the project “Advanced neuroimaging to study aging”.
Disclosure
Tobias Kober is an employee of Siemens Healthineers International AG Switzerland, owns stocks of Siemens Healthineers and holds patents filed by Siemens Healthineers.
References
- Sherman SM. Functioning of Circuits Connecting Thalamus and Cortex. Comprehensive Physiology. Published online March 16, 2017:713-739. doi:10.1002/cphy.c160032
- Wang F, Lai Y, Pan Y, Li H, Liu Q, Sun B. A systematic review of brain morphometry related to deep brain stimulation outcome in Parkinson’s disease. npj Parkinson’s Disease. 2022;8(1). doi:10.1038/s41531-022-00403-x
- Vetkas A, Fomenko A, Germann J, et al. Deep brain stimulation targets in epilepsy: Systematic review and meta‐analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia. 2022;63(3):513-524. doi:10.1111/epi.17157
- Power BD, Looi JC. The thalamus as a putative biomarker in neurodegenerative disorders. Australian & New Zealand Journal of Psychiatry. 2015;49(6):502-518. doi:10.1177/0004867415585857
- Hollingsworth KG. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Physics in Medicine & Biology. 2015;60(21):R297
- Mussard E, Hilbert T, Forman C, Meuli R, Thiran J, Kober T. Accelerated MP2RAGE imaging using Cartesian phyllotaxis readout and compressed sensing reconstruction. Magnetic Resonance in Medicine. 2020;84(4):1881-1894. doi:10.1002/mrm.28244
- Mair RW, Hanford LC, Mussard E, Hilbert T, Kober T, Buckner RL. Optimizing rapid compressed-sensing MPRAGE acquisitions for repeat sampling of brain morphometry within individuals. Proceedings of the International Society for Magnetic Resonance in Medicine. 2020;28:0564.
- Dieckmeyer M, Roy AG, Senapati J, et al. Effect of MRI acquisition acceleration via compressed sensing and parallel imaging on brain volumetry. Magnetic Resonance Materials in Physics, Biology and Medicine. 2021;34(4):487-497. doi:10.1007/s10334-020-00906-9
- Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage. 2018;183:314-326. doi:10.1016/j.neuroimage.2018.08.012
- O’Brien KR, Kober T, Hagmann P, et al. Robust T1-Weighted Structural Brain Imaging and Morphometry at 7T Using MP2RAGE. Margulies D, ed. PLoS ONE. 2014;9(6):e99676. doi:10.1371/journal.pone.0099676
- Mair RW, Hanford LC, Mussard E, Hilbert T, Kober T, and Buckner RL. Towards 1 min brain morphometry - evaluating compressed-sensing MPRAGE. Proceedings of the International Society of Magnetic Resonance in Medicine. 2019;27:2978.
- Shin KJ, Lee H-J, Park KM. Alterations of individual thalamic nuclei volumes in patients with migraine. The Journal of Headache and Pain. 2019;20(112).
- Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Multiple Sclerosis Journal. 2013;19(11):1478-1484. doi:10.1177/1352458513478675
- Schoonheim MM, Hulst HE, Brandt RB, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology. 2015;84(8):776-783. doi:10.1212/wnl.0000000000001285
Figures
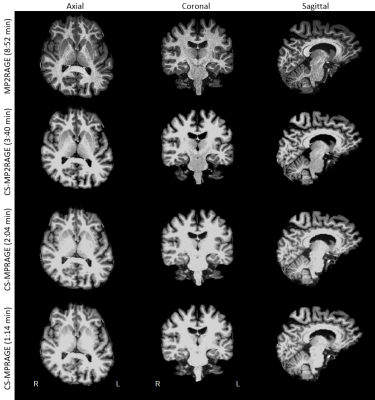
Figure 1. FreeSurfer-extracted brain of a representative subject across MPRAGE sequence variants for qualitative comparisons. Images are shown with different brightness settings to better illustrate tissue contrast, which is well preserved for all sequences. Sagittal images show the right hemisphere. Abbreviations as in text.
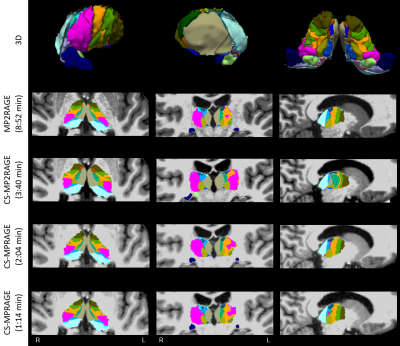
Figure 2. FreeSurfer automated thalamic parcellations of a representative sample subject in the considered sequences, showing image contrast differences across sequences. Top row: 3D rendering of lateral (left), medial (middle), and ventral (right) aspects of thalamic nuclei from MP2RAGE data. Nuclei labels and colors as in [9]. Sagittal images show the right hemisphere. Abbreviations as in text.
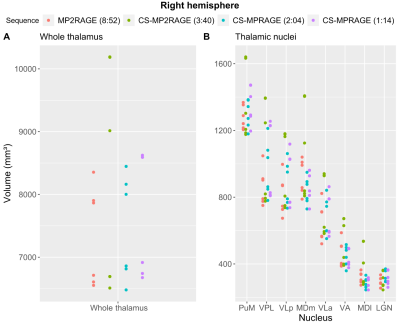
Figure 3. Distribution of the volumes of the six sample subjects as a function of sequence. (A): right-hemisphere whole thalamus; (B): right-hemisphere major thalamic nuclei sorted by descending order of volume. For each sequence in the legend, TA are in brackets. Abbreviations as in text.
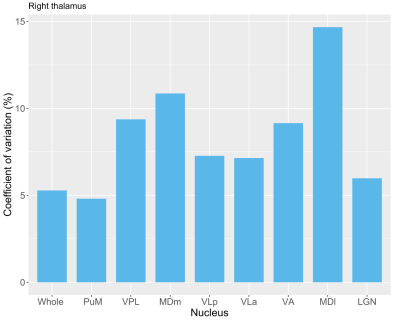
Figure 4. Volume variation of right-hemisphere whole thalamus and major thalamic nuclei across sequences. Nuclei are sorted by descending order of volume, as in Fig. 3. Abbreviations as in text.
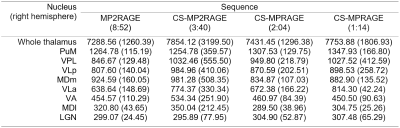
Table 1. Median (interquartile range) volume (mm3) of the right-hemisphere whole thalamus and major thalamic nuclei in the considered sequences (N=6 healthy subjects). For each sequence, TA are in brackets. Abbreviations as in text.
DOI: https://doi.org/10.58530/2023/3588