3572
QSM and R2* relaxometry analysis for mapping of white matter hyptension and cognition in patients with lacunar Infarction1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Neurology, The Affiliated Zhongshan Hospital of Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China
Synopsis
Keywords: Vessels, Perfusion
Due to the lacunar infarcts are small, they are asymptomatic in most cases. However, the location and accumulation of multiple lacunar infarcts might lead to severe physical and cognitive impairment. This lesion, caused by the involvement of cerebral small vessel in the brain, is thought to be associated with an increased risk of Vessel dementia (VaD). Therefore, exploring the perfusion alterations in patients with early lacunar infarction might help with interventions and preventive measures, thereby reducing the social and healthcare burden.Introduction
Cerebral small vessel disease (CSVD) is a widespread cerebrovascular disease that affects small arteries, arterioles and capillaries of the brain1. The prevalence of CSVD is currently as high as 3% to 8% worldwide, with an increasing trend in the aging population2. It is primarily responsible for stroke incidents, gait disturbances, depression, cognitive impairment, and dementia in the elderly3. Currently, the detection of CSVD mainly relies on MRI markers, including lacune of presumed vascular origin, small subcortical infarct, white matter hyperintensities (WMHs), perivascular spaces (PVS), cerebral microbleeds (CMBs), lacunar infarction (LI) and brain atrophy4,5. It has been shown that WMHs and subcortical infarcts of CSVD are accompanied by reduced brain perfusion6,7. In addition, Studies have shown increased iron deposition in areas of vascular damage in VaD patients8, and both clinical and basic studies have shown abnormal iron deposition in the hippocampus and nucleus accumbens in VaD patients or animal models and a close correlation with neuropsychological assessments9, suggesting that abnormal iron deposition in the brain may be involved in the development of cognitive impairment in VaD. However, there are few studies measuring iron deposition in early LI. Therefore, it is important to determine the relationship between early iron deposition and cognition of LI. We analysed the relationship between white matter hypertension and cognition with iron deposition and R2* in LI patients. The large variability in clinical symptoms among patients with LI can probably be understood by taking into account the heterogeneity of LI lesions and the effects of LI beyond the focal lesions.Methods
SubjectsThe study population consisted of 46 (15 male and 31 female) consecutive patients with single and disseminated LI admitted to the Department of Neurology of the Zhongshan Hospital, in Shanghai, China. Patients with stroke history or intracerebral hemorrhage were excluded from the study as were those with severe cardiovascular disease, renal insufficiency, liver dysfunction, neoplastic or chronic disease, major depression and other psychiatric comorbidity (DSM-IV-R). A battery of neuropsychological tests was administered to all patients within 1 month after MRI scanning.
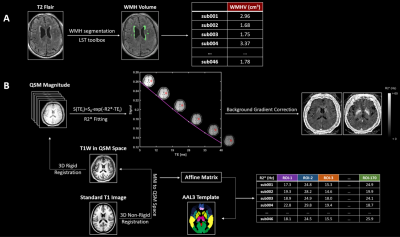
Image acquisition
MRI studies were performed using a Philips 3.0 Tesla Ingenia system and the protocol included 3D T1W_TFE, 3D_T2_TSE, 3D T2_FLAIR. A 3D multi-echo GRE sequence was used to acquire quantitative susceptibility mapping (QSM): TR=43.0ms; number of echoes=6; first TE=5ms; TE spacing=7ms; flip angle=17°; FOV = 230mm×188mm; spatial resolution=0.34×0.34×0.75mm; CS-SENSE factor=4.
Image processing
The QSM maps were calculated using STISuite toolbox. For R2* maps, as shown in Figure 1B, the corrections for the background fields were performed with phase images10, and then the QSM magnitude images were used to generate R2* estimates using fast mono-exponential fitting algorithm of all 6 echoes11. The T1 and FLAIR images were aligned to DARTEL space, and then the WMH segmentation was performed with the k-NN algorithm. Software (SPSS version 26.0; SPSS, USA) was employed for the correlation between MRI Indicators (WMHV, R2*, QSM) and neuropsychological assessments of 170 brain regions (Automated anatomical labelling atlas 3)12. A significant difference was present if the p-value was less than 0.01.
Results
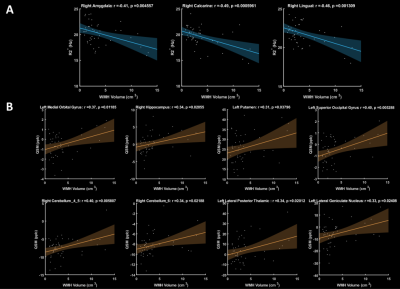
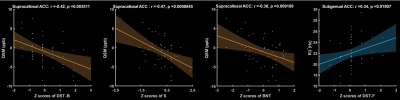
The R2* of right amygdala, right calcarine and right lingual were found to be negatively correlated with their WHM volume (Fig. 2). As more iron is deposited, the white matter lesions increased. As Fig. 2 demonstrates, QSM in left medial orbital gyrus, right hippocampus, left putamen, left superior occipital gyrus and left lateral posterior thalamic all correlate positively with the WMH volume. It is worth noting (Figure 3) that we found some interesting results in Supracallosal ACC. The QSM of Supracallosal ACC was found to be negatively correlated with digital span test - backward, Similarity test and Boston naming test. However, the QSM of Supracallosal ACC was positively correlated with digital span test – forward.Discussion and conclusion
In this study on patients with LI, we assessed the relationship between whole brain R2* and iron deposition and white matter high signal volume. We found that R2* were was correlated associated with WMM volume in patients with LI. When there is more white matter damage, then there is more iron deposition in the corresponding brain region and less blood oxygenation, which is consistent with most studies. In this study, the correlation of R2* in amygdala with WHM volume was reflective of cognitive dysfunction in dementia. The amygdala is an important structure for emotional learning and memory13. Moreover, the association of R2* changes with cognitive performance in Supracallosal ACC might be appropriate for the early evaluation of cognitive decline in VaD. The Supracallosal ACC, in which digital span test – forward was found to have significant correlation with R2*, is associated with verbal short-term memory and working memory14,15. As the R2* value was correlated with the decline of cognitive function, the R2* value may potentially be an emerging biomarker for assessing the progression of cognitive impairment in patients LI. In the brain, iron was involved in synthesis of neurotransmitters and myelin formation, iron deposition played a key role in the pathogenesis of CNS16. Furthermore, most studies show that WMH might causes cognitive decline. In our study, we found that the content of iron in hippocampus was increased when WMH volume increased. It was indicated that excessive iron had deposited in hippocampus after chronic cerebral hypoperfusion, and iron deposition could trigger cognitive impairment.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 81971583), National Key R&D Program of China (No. 2018YFC1312900), Shanghai Municipal Science and Technology Major Project (No. 20ZR1406400) and ZJLab.References
1. Li Q, Yang Y, Reis C, et al. Cerebral small vessel disease. Cell Transplant. 2018;27: 1711-1722.
2. Wardlaw JM., Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol. 2020;16: 137-153.
3.Chojdak-Lukasiewicz J, et al. Cerebral small vessel disease: A review. Advances in clinical and experimental medicine. 2021;30(3): 349-356.
4. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9: 689-701.
5. Chen X, Wang J, Shan Y, et al. Cerebral small vessel disease: neuroimaging markers and clinical implication. J. Neurol.2019;266: 2347-2362.
6. Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain, 2002;125:1094-1104.
7. Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63: 246-253
8. Kalaria RN, et al. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathological. 2016;131(5): 659-685.
9. Zecca L, et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proceeding of the National Academy of Sciences of USA. 2004;101(26): 9843-9848.
10.Rapid single-scan T2*-mapping using exponential excitation pulses and image-based correction for linear background gradients doi: 10.1002/mrm.21971.
11.Algorithm for fast mono-exponential fitting based on Auto-Regression on Linear Operations (ARLO) of data doi: 10.1002/mrm.25137
12.Rolls ET, Huang CC, et al. Automated anatomical labelling atlas 3. Neuroimaging. 2020;206:1-5.
13.Yang Y, and Wang JZ. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Frontiers in Neural Circuits. 2017;31.
14.Majerus S. Language repetition and short-term memory: an integrative framework. Frontiers in Human Neuroscience. 2013; 12.
15.Schwering SC, and MacDonald MC. Verbal Working Memory as Emergent from Language Comprehension and Production. Frontiers in Human Neuroscience. 2020; 14:68.
16.Huo T, Jia Y, et al. Iron dysregulation in vascular dementia: Focused on the AMPK/autophagy pathway. Brain Research Bulletin. 2019; 153:305-313.