3569
BTX: Simultaneous 3D Quantitative Magnetization Transfer Imaging and Susceptibility Mapping1Athinoula A. Martinos Center for Biomedical Imaging, Harvard Medical School, Charlestown, MA, United States, 2Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Radiology, University of California, San Diego, San Diego, CA, United States
Synopsis
Keywords: Quantitative Imaging, Multi-Contrast, Magnetization Transfer, Quantitative Susceptibility Mapping
We propose a novel sequence that enables simultaneous quantitative magnetization transfer (qMT) imaging and susceptibility mapping (QSM) for assessing tissue composition, microstructure, and microenvironment. We extend our BTS sequence to incorporate a multi-echo acquisition scheme for probing tissue susceptibility information. The acquisition allows quantification of B1+, bias-corrected T1, qMT parameters (macromolecule bound proton fraction and proton exchange rate), tissue susceptibility and T2*. The method’s feasibility is demonstrated using an in-vivo brain scan, where 3D concurrent qMT and QSM were obtained for the whole brain at feasible scan time.Introduction
Both quantitative magnetization transfer (qMT) imaging1 and susceptibility mapping (QSM)2 can provide promising imaging biomarkers for evaluating tissue pathogenesis in neural3,4, cardiac5,6, body7,8 and musculoskeletal diseases9,10. While each technique utilizes separate modeling of MR signal magnitude (for qMT) and phase (for QSM) to obtain subsequent quantitative information, there has not been a single acquisition strategy and modeling technique that can assess both in a unified framework. Measuring qMT and QSM simultaneously has not yet been well-established in applications such as multiple sclerosis and osteoarthritis assessment in the brain and knee. Recently, we proposed a new method BTS11 (Bloch-Siegert and magnetization Transfer Simultaneously) for qMT imaging using a spoiled gradient-echo sequence with off-resonance RFs to model Bloch-Siegert12 shift (for B1+ correction) and MT13. In this work, we extend our method (denoted BTX) to incorporate a multi-echo acquisition for obtaining QSM parameters. This new sequence, in conjunction with our modeling technique, leverages both the magnitude and phase of the acquired MR signals, enabling simultaneous qMT and QSM at no cost in additional scan time.Theory
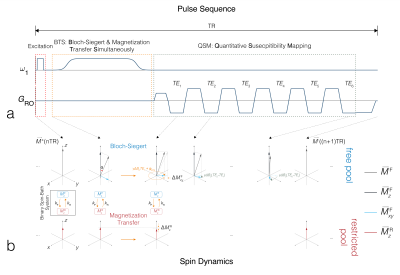
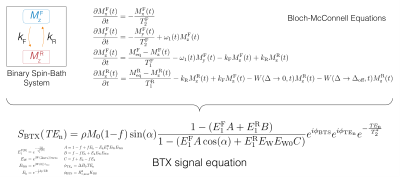
BTS: The original BTS sequence is implemented on a spoiled gradient-echo scheme where an off-resonance RF is introduced between excitation and acquisition (FIG1A). This induces two independent effects: 1) MT through direct saturation of macromolecules and 2) B1+-dependent Bloch-Siegert phase shift of free water proton. The BTS term in FIG3A gives the resulting analytical signal equation. Using a variable flip-angle (vFA) scheme with (BTS) and without (baseline) off-resonance RF applied, this signal term is used to generate B1+ and T1F (MT-corrected T1 for free water) maps and MT parameter (macromolecule proton fraction f and exchange rate kF) maps. New BTX: Our proposed BTX (BTS with susceptibility mapping) utilizes the idle time between excitation and acquisition for a multi-echo acquisition module (FIG1A). This enables T2* quantification via magnitude decay and magnetic susceptibility (χ) quantification via spin phase evolution governed by the additional T2* and QSM terms in FIG3A. As a result, this unified sequence and modeling allows quantification of B1+ (for flip-angle correction), free water T1F, MT parameters f and kF, tissue susceptibility χ and T2*, concurrently, at no additional scan time.Methods
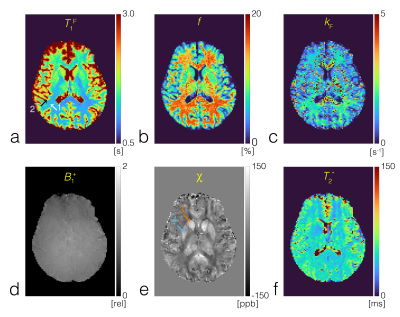
An in-vivo experiment was conducted to scan the whole sagittal brain of a healthy volunteer using a 32-channel head coil on a 3T scanner (Siemens Prisma). Sequence parameters include: field of view = 23x23x16cm3, matrix size = 174x176x80, slice thickness = 2mm, number of slices = 80, total 6 TEs spanning 11ms to 36ms with 5ms echo spacing, TR = 40ms. Multiple scans were acquired at varying prescribed flip-angles of 5˚, 20˚ and 40˚, with an 8ms fermi pulse at off-resonance frequency 4kHz and effective flip-angle 629˚ used for off-resonance saturation, turned on and off. An additional acquisition at flip-angle 20˚ with off-resonance frequency -4kHz was also acquired for B1+ map estimation using the Bloch-Siegert method12. Utility of signal magnitude: The magnitude of MR signals and actual flip-angles extracted from B1+ maps were then fit into the BTX model to generate T1F, f and kF (FIG3B) under the assumption of fixed MT properties for macromolecule bound proton T1R = 1s, T2R = 12μs and a Super-Lorentzian MT saturation line shape14. T2* was also estimated by fitting an exponential decay as a function of TEs (FIG3D). Utility of signal phase: The phase of MR signals at multiple echoes was used to estimate the total field15, which was subsequently used in projection onto dipole (PDF)16 method to remove the background field. The estimated tissue field was then used to estimate susceptibility χ using morphology-enabled dipole inversion (MEDI)17 (FIG3C). Inter-scan motion was compensated using a co-registration method18 and images were reformatted into axial view to better represent all maps in the thalamus brain region.Results and Discussion
Quantitative MT (FIG4A-C), B1+ (FIG4D), QSM (FIG4E) and T2* (FIG4F) maps of the in-vivo brain measured concurrently are presented. T1F, MT parameters f and kF, magnetic susceptibility χ and T2* were measured in the white matter and gray matter ROI regions indicated by the numbered white and black arrows in FIG4A, and the caudate and putamen region indicated by the numbered orange and blue arrows in FIG4E. T1F, f, kF, χ and T2* for white matter (1): 0.992[s]/1.158[s]/15.0[%]/1.63[s-1]/-19.7[ppb]/45[ms]; gray matter (2): 1.231[s]/1.432[s]/11.2[%]/1.2[s-1]/16.2[ppb]/37[ms]; caudate (3): 1.597[s]/1.843[s]/7.8[%]/0.9[s-1]/74.3[ppb]/41.6[ms]; putamen (4):1.415[s]/1.550[s]/6.7[%]/1.7[s-1]/74.3[ppb]/38.6[ms]. Measured values correspond with values reported in literature1,19.Conclusion
BTX, a new unified sequence and modeling technique that simultaneously provides magnetization transfer and susceptibility quantification, has been proposed. Simultaneous estimation of B1+ for compensation, free water T1F, MT parameters (f and kF) reflecting macromolecule content, magnetic susceptibility χ and T2* was demonstrated in-vivo brain, showing great agreements with reported values from separate qMT and QSM experiments1,19. The ability to simultaneously obtain qMT and QSM biomarkers can provide complementary information to facilitate assessment of tissue composition, microstructure, and microenvironment in multiple sclerosis with myelin damage and iron deposition in the brain, and osteoarthritis with cartilage extracellular matrix degeneration and microcalcification in the joint. As a result, our proposed method will provide powerful tools for improving the understanding of complex pathophysiology in various neural, cardiac, body and musculoskeletal diseases.Acknowledgements
We thank the funding support from NIBIB R21EB031185, NIAMS R01AR079442, R01AR081344 and R01AR078877.References
1. Yarnykh VL, Yuan C. Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human brain. Neuroimage 2004;23:409-424.
2. Wang Y, Liu T. Quantitative Susceptibility Mapping (QSM): Decoding MRI Data for a Tissue Magnetic Biomarker. Magn Reson Med 2015;73:82-101.
3. Vymazal J, Righini A, Brooks RA, Canesi M, Mariani C, Leonardi M, Pezzoli G. T1 and T2 in the Brain of Healthy Subjects, Patients with Parkinson Disease, and Patients with Multiple System Atrophy: Relation to Iron Content. Radiology 1999;211:489-495.
4. Chen W, Gauthier SA, Gupta A, Comunale J, Liu T, Wang S, Pei M, Pitt D, Wang Y. Quantitative Susceptibility Mapping of Multiple Sclerosis Lesions at Various Ages. Radiology 2014;271:183-192.
5. Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2003;(2):353-359.
6. Wen Y, Nguyen TD, Liu Z, Spincemaille P, Zhou D, Dimov A, Kee Y, Deh K, Kim J, Weinsaft JW, Wang Y. Cardiac Quantitative Susceptibility Mapping (QSM) for Heart Chamber Oxygenation. Magn Reson Med 2018;79(3):1545-1552.
7. Luetkens JA, Klein S, Träber F, Schmeel FC, Sprinkart AM, Kuetting DLR, Block W, Uschner FE, Schierwagen R, Hittatiya K, Kristiansen G, Gieseke J, Schild HH, Trebicka J, Kukuk GM. Quantification of Liver Fibrosis at T1 andT2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018;288(3):748-754.
8. Sharma SD, Hernando D, Horng DE, Reeder SB. Quantitative Susceptibility Mapping in the Abdomen as an Imaging Biomarker of Hepatic Iron Overload. Magn Reson Med 2015;74(3):673-83.
9. Mittal S, Pradhan G, Singh S, Batra R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol J Radiol 2019;84:e549-e564.
10. Jang H, von Drygalski A, Wong J, Zhou JY, Aguero P, Lu X, Cheng X, Ball ST, Ma Y, Chang EY, Du J. Ultrashort echo time quantitative susceptibility mapping (UTE-QSM) for detection of hemosiderin deposition in hemophilic arthropathy: A feasibility study. Magn Reson Med 2020;84(6):3246-3255.
11. Jang A, Han PK, Ma C, El Fakhri G, Liu F. Bias-free T1 Mapping via Simultaneously Estimating Bloch-Siegert and Magnetization Transfer Effects. ISMRM 2022, London, UK.
12. Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B1 Mapping by Bloch-Siegert Shift. Magn Reson Med 2010;63:1315–1322.
13. Wolff SD, Balaban RS. Magnetization Transfer Contrast (MTC) and Tissue Water Proton Relaxation in Vivo. Magn Reson Med 1989;10:135-144.
14. Morrison C, Henkelman RM. A Model for Magnetization Transfer in Tissues. Magn Reson Med 1995;33:475-482.
15. de Rochefort L, Brown R, Prince MR, Wang Y. Quantitative MR Susceptibility Mapping Using Piece- Wise Constant Regularized Inversion of the Magnetic Field. Magn Reson Med 2008;60(4):1003-1009.
16. Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, Wang Y. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed 2011;24(9):1129–36.
17. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59(3):2560-2568.
18. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. elastix: A Toolbox for Intensity-BasedMedical Image Registration. IEEE Trans Med Imaging 2010;29(1):196-205.
19. Li W Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage 2011;55:1645-1656.
Figures