3566
Quantitative evaluation of deep gray matter in striatal-cerebellar-brainstem circuits in Parkinson’s disease patients with freezing of gait1Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Department of Radiology, Wayne State University, Detroit, MI, United States
Synopsis
Keywords: Quantitative Imaging, Quantitative Susceptibility mapping
This study evaluated the structural changes of the deep gray matter in the cortico-basal ganglia and cerebellar motor circuit in Parkinson’s disease patients with freezing of gait. Using region-of-interest-based quantitative susceptibility mapping analysis, we found pronounced striatum atrophy combined with caudal substantia nigra abnormal iron accumulation in Parkinson’s disease patients with freezing of gait. This result offered new insight into future research investigating the pathophysiology of the freezing of gait.Introduction
Freezing of gait (FOG) is a common and disabling gait difficulty in Parkinson’s disease (PD)1. The occurrence of FOG is associated with the dorsal cortico-basal ganglia and cerebellar motor circuits2. The structural impairments especially the deep gray matter (DGM) in Parkinson’s disease (PD) patients with FOG have been discussed extensively but the structures triggering FOG remain elusive. This study aimed to examine the mean volume and iron accumulation of DGM in striatal- cerebellar-brainstem neural circuits in PD patients with FOG using region-of-interest-based quantitative susceptibility mapping (QSM) analysis.Method
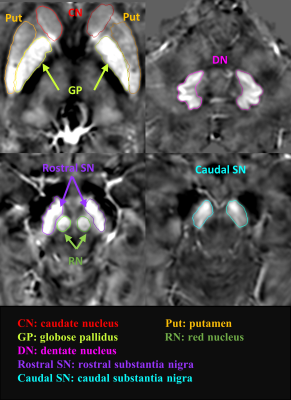
Twenty (20) PD patients with FOG (PD-FOG), 19 PD patients without FOG (PD-nFOG), and 30 age- and sex-matched healthy controls (HCs) were scanned on a 3T Philips MRI system using a 3D multi-echo gradient recalled echo to acquire QSM data. The imaging parameters were as follows: TR = 45 ms, sixteen echoes with TE1= 3.2 ms, ΔTE = 2.6 ms, bandwidth = 541 Hz/pixel, flip angle = 12◦, field of view (FOV) = 220 mm, matrix size = 256 × 256, resolution = 0.86 × 0.86 × 1.0 mm3. Fourteen regions of interest (ROIs) (Figure 1) including the bilateral caudate head, putamen, globus pallidus, red nucleus, dentate nucleus, rostral substantia nigra, and caudal substantia nigra were automatically segmented on QSM maps, respectively, using SPIN software (SpinTech, Inc., Bingham Farms, Ml, USA) with dynamic programming algorithm3. Then we simultaneously measured DGM volume and iron deposition on the QSM data. Intergroup difference analysis was performed using IBM SPSS Statistics (version 26; IBM Corp., Armonk, NY).Results
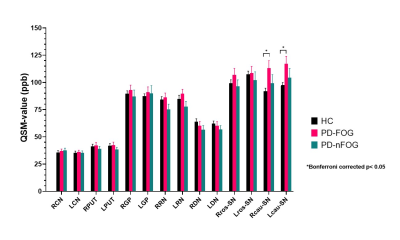
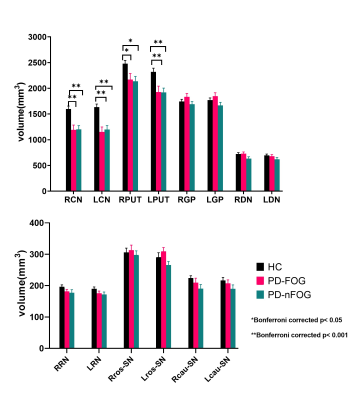
Figure 2 shows intergroup comparison results of DGM volume and iron content between PD-FOG, PD-nFOG, and HC. Both the PD-FOG and PD-nFOG groups had significantly lower volumes than the HCs group in the bilateral caudate head (all p< 0.001) and putamen (right p< 0.05 and left p< 0.001). The PD-FOG group had higher QSM values in the bilateral caudal substantia nigra than the HCs group (p< 0.05 with Bonferroni corrected).Discussion
Consistent with the previous research4, the PD-FOG group showed significantly higher iron accumulation in the caudal substantia nigra compared to the HC, whereas we showed no difference in the SN iron content between PD-nFOG and HC groups. This caudal substantia nigra territory is known to show bilateral and unilateral loss of the nigrosome-1 sign in PD subjects, therefore, in these cases, there should be an increase in iron content, however, not all PD subjects have high SN iron deposition5. Atrophy of the bilateral caudate head and putamen observed in this study was consistent with several previous investigations of volumes of whole-brain gray matter in FOG individuals6, 7. But we observed the same changes in PD-nFOG subjects. The caudate head and putamen are enriched with dopamine receptors8 and receive dopaminergic signals from the substantia nigra compacta. Therefore, any atrophy is expected to lead to a reduction of dopamine receptors. Although we found the atrophy of the bilateral caudate head and putamen whether PD-FOG or PD-nFOG, only the PD-FOG group showed abnormal caudal substantia nigra iron deposition. Therefore, the atrophy of the striatum combined with increased iron content in the caudal substantia nigra might be responsible for FOG.Conclusion
In this study, we focused on the structural changes of DGM in the striatal-cerebellar-brainstem neural circuits and found the decreased volume in the striatum and increased iron deposition in caudal substantia nigra both appeared in PD-FOG subjects. These changes may alter the function of the substantia nigra pars reticulata, the main output nucleus of the basal ganglia pathway resulting in gait difficulty. Our results offered new insight into future research investigating the pathophysiology of the freezing of gait.Acknowledgements
No acknowledgement found.References
1. Perez-Lloret S, Negre-Pages L, Damier P, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol 2014;71(7):884-890.
2. Gilat M, Ginis P, Zoetewei D, et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson's disease. NPJ Parkinsons Dis 2021;7(1):81.
3. Jiang J, Haacke EM, Dong M. Dependence of vessel area accuracy and precision as a function of MR imaging parameters and boundary detection algorithm. Journal of Magnetic Resonance Imaging 2007;25(6):1226-1234.
4. Naduthota RM, Honnedevasthana AA, Lenka A, et al. Association of freezing of gait with nigral iron accumulation in patients with Parkinson's disease. J Neurol Sci 2017;382:61-65.
5. He N, Ghassaban K, Huang P, et al. Imaging iron and neuromelanin simultaneously using a single 3D gradient echo magnetization transfer sequence: Combining neuromelanin, iron and the nigrosome-1 sign as complementary imaging biomarkers in early stage Parkinson's disease. Neuroimage 2021;230:117810. 6. Kostic VS, Agosta F, Pievani M, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology 2012;78(6):409-416.
7. Herman T, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM. Gray matter atrophy and freezing of gait in Parkinson's disease: Is the evidence black-on-white? Mov Disord 2014;29(1):134-139.
8. Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990;250(4986):1429-1432.
Figures