3560
A Multimodal Atlas of the Human Cerebellum at 760 μm Resolution1Radiology and Biomedical Research Imaging Center (BRIC), The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Keywords: Multimodal, Multimodal, Cerebellum; atlas;
The human cerebellum is engaged in a broad array of tasks related to motor coordination, cognition, and emotional regulation. Here, we construct a cerebellar atlas using high-resolution multimodal MRI, capturing multiple characteristics of the cerebellum, including cortical morphology, tissue microstructure, and cerebellar/cerebello-cerebral connectivity. Our atlas facilitates the understanding of the neurodevelopment and neurodegeneration of the cerebellum.Introduction
The human cerebellum is engaged in a broad array of tasks related to motor coordination, cognition, language, attention, memory, and emotional regulation 1-3. A detailed cerebellar atlas can facilitate the investigation of the structural and functional organization of the cerebellum. However, existing cerebellar atlases are typically limited to a single imaging modality with insufficient characterization of tissue properties. Here, we introduce a multimodal cerebellar atlas based on high-resolution multimodal MRI.Methods
MRI data was acquired at submillimeter isotropic resolutions for a young male using the MGH-USC 3T CONNECTOM scanner with a 64-channel coil 4 and the following acquisition parameters:- T1-weighted (T1w) MRI: repetition time (TR) = 2530 ms; slice thickness = 0.7 mm; FOV = $$$256 \times 256 \times 146\,\text{mm}^3$$$.
- Diffusion-weighted MRI: TR = 3500 ms; TE = 75 ms; in-plane resolution = 0.76 mm; slice thickness = 0.76 mm; FOV = $$$220 \times 288\,\text{mm}^2$$$; b-values = 0, 1000, 2500 $$$\text{s}/\text{mm}^2$$$ for 144, 420, 840 directions, respectively.
We quantified cerebellar tissue microstructure with Spherical Mean Spectrum Imaging (SMSI) 6,7, giving microscopic fractional anisotropy and microscopic mean diffusivity as well as volume fractions of free-water, intra-soma, intra-axonal, and extra-cellular compartments.
Tractography was performed using asymmetric fiber orientation distribution functions (AFODFs) to better capture complex axonal configurations and to mitigate gyral bias for better corticocortical connectivity 8. Fiber streamlines were generated by successively following the local directions determined from the AFODFs. Whole-brain tractography was performed with 64 random seeds per voxel, resulting in approximately 100 million streamlines, but only streamlines that terminated at the cerebellar cortical regions were retained. We employed a recent approach 9 to identify a total of $$$M=\{4,8,12,16,20,24,28,32,36,38\}$$$ parcels from $$$H=\{50,100,150,200,250,300,350,400,450,500\}$$$ bundles generated by unsupervised fiber clustering of the cerebellum tractogram, resulting in the $$$10 \times 10$$$ initial label maps. Multi-scale consistent parcellation was then performed by joint consideration of all of the initial label maps over all $$$M$$$'s and $$$H$$$'s. We manually selected the best parcellation $$$\bar{M}=25$$$ from all of the candidate parcellations with $$$\bar{M}=4$$$ to $$$\bar{M}=100$$$, and corrected these parcellations based on previous studies 10-12.
Results
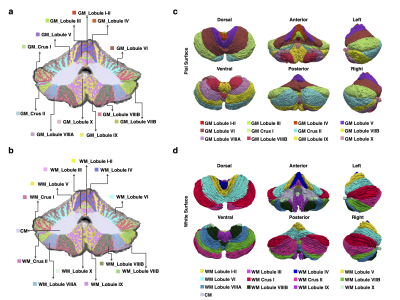
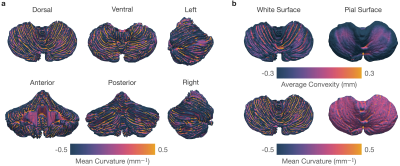
We parcellated each cerebellar hemisphere into 13 subregions: CM, Lobule I-II, Lobule III, Lobule IV, Lobule V, Lobule VI, Crus I, Crus II, Lobule VIIB, Lobule VIIIA, Lobule VIIIB, Lobule IX, and Lobule X. Except for CM, each lobule was subdivided into GM and WM. (Figures 1a-b).The reconstructed pial and white surfaces of the cerebellar cortex are shown in Figures 1 c-d and their average convexity and mean curvature maps are shown in Figure 2. Mean curvature captures fine-scale folding patterns of folia and fissures, whereas average convexity captures coarse-scale folding patterns at lobule level. Both global and local folding patterns are preserved by the reconstructed cerebellar surfaces. The intricate folding patterns of the folia and fissures are evident from the mean curvature map shown in Figure 2a.
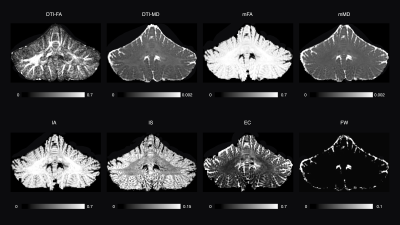
SMSI gives meaningful maps of tissue cerebellar microstructure (Figure 3). Notably, the intra-axonal volume fraction map highlights the WM, whereas the intra-soma map highlights the cerebellar GM, which consists of organized and densely packed granule cells and Purkinje cell bodies.
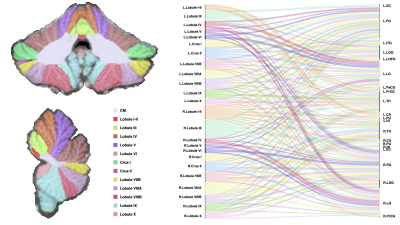
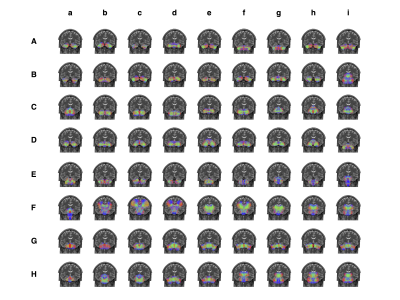
We investigated the structural connectivity of each cerebellar region to different cerebral regions (Figure 4). The results indicate that bilateral Lobule III, Crus II, Lobule VIIB, right Lobule I-II, and right Lobule VIIIA are robustly connected to multiple regions of the cerebrum. We identified 44 intracerebellar bundles (Figure 5 Aa-Eh), involving almost all cerebellar regions; 2 bundles between the cerebellum and the medulla (Figure 5 Ei, FA); 14 bundles between the cerebellum and the pons (Figure 5 Fh-He); 4 bundles between the cerebellum and the thalamus (Figure 5 Hf-Hi); 2 bundles between the cerebellum and the basal ganglia; and 6 bundles between the cerebellum and various regions of the cerebral cortex (Figure 5 Fb-Fg).
Discussion
In this work, we constructed a high-resolution multimodal atlas that captures morphological, microstructural, and connectivity features of the cerebellum. To date, few studies considered the geometric complexity of the cerebellar cortex.The cerebellar surfaces of our atlas capture the fine-scale folding patterns of folia and fissures, allowing the investigation of surface morphology with respect to cerebellar functions. Our atlas is enriched by information on tissue microstructure, allowing fine-grained characterization of subvoxel tissue compartments. Our atlas also captures information on cerebellar and cerebello-cerebral connectivity, facilitating the understanding of the role of the cerebellum in functions such as motor coordination and cognition.
Acknowledgements
This work was supported in part by the United States National Institutes of Health (NIH) under grants MH125479, EB008374, and EB006733.References
1. Stoodley, C.J., The cerebellum and cognition: evidence from functional imaging studies. Cerebellum, 2012. 11(2): p. 352-65.
2. Turner, B.M., et al., The cerebellum and emotional experience. Neuropsychologia, 2007. 45(6): p. 1331-41.
3. Timmann, D., et al., The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex, 2010. 46(7): p. 845-57.
4. Wang, F., et al., In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Scientific Data, 2021. 8(1): p. 1-12.
5. Fischl B. FreeSurfer. Neuroimage, 2012, 62(2): 774-781.
6. Huynh, K.M., et al. Characterizing intra-soma diffusion with spherical mean spectrum imaging. in International Conference on Medical Image Computing and Computer-Assisted Intervention. 2020. Springer.
7. Huynh, K.M., et al., Probing tissue microarchitecture of the baby brain via spherical mean spectrum imaging. IEEE transactions on medical imaging, 2020. 39(11): p. 3607-3618.
8. Wu, Y., et al., Mitigating gyral bias in cortical tractography via asymmetric fiber orientation distributions. Medical image analysis, 2020. 59: p. 101543.
9. Wu, Y., et al., Highly Reproducible Whole Brain Parcellation in Individuals via Voxel Annotation with Fiber Clusters. 2021. Cham: Springer International Publishing.
10. Han, S., et al., Hierarchical Parcellation of the Cerebellum. Med Image Comput Comput Assist Interv, 2019. 11766: p. 484-491.
11. Romero, J.E., et al., CERES: A new cerebellum lobule segmentation method. Neuroimage, 2017. 147: p. 916-924.
12. Schmahmann, J.D., et al., Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage, 1999. 10(3): p. 233-260.
Figures