3559
Stereotactic MRI-guided radiosurgery using AI resting state networks recognition1Instituto Balseiro, Universidad Nacional de Cuyo, Bariloche, Argentina, 2Universidad de Mendoza, Mendoza, Argentina, 3Fundación Escuela de Medicina Nuclear, Mendoza, Argentina, 4Fundacion Argentina para el Desarollo en Salud, Mendoza, Argentina, 5Harvard Medical School, Boston, MA, United States, 6Fundación Argentina para el Desarrollo en Salud, Mendoza, Argentina
Synopsis
Keywords: Tumors, Radiotherapy
Stereotactic radiosurgery (SRS) is a minimally invasive procedure that reduces tumor size without subjecting surrounding organs at risk (OAR) to harmful radiation levels. The delimitation of OAR in SRS planning, and the conservation of the prescribed dose in tumoral regions, can be improved by the implementation of independent component analysis to obtain resting-state fMRI networks (RSNs). In this aspect, AI plays an important role to decrease computational-processing times while increasing the method’s efficiency. One of the main solved challenges was the lack of compatibility between the Nifti protocol (RSNs format) and the treatment planning system’s protocol (coordinate system of CT-simulation).Introduction
Stereotactic Radiosurgery (SRS) is a non-invasive technique that uses multiple converging beams delivering high doses to the target in a single fraction. In the brain, this technique is usually employed to treat injuries smaller than 3 cm located in deep and eloquent regions of the brain1. The doses received by some functional regions, such as the ones detected by resting-state fMRI (RSNs), can be reduced through the incorporation of those regions as organs at risk (OAR) in the radiotherapy treatment planning systems (RTPS).The aim of this project is to recognize and incorporate the RSNs into the treatment planning process to minimize the radiation doses in these OAR, while reducing computational-processing times and increasing the efficiency through methodologies like AI-based RSNs recognition.Methodology
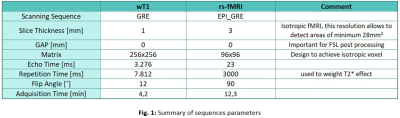
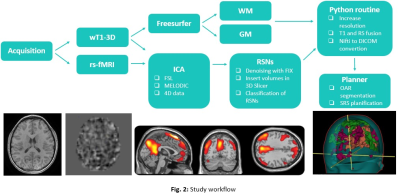
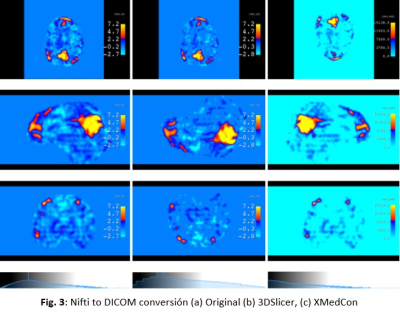
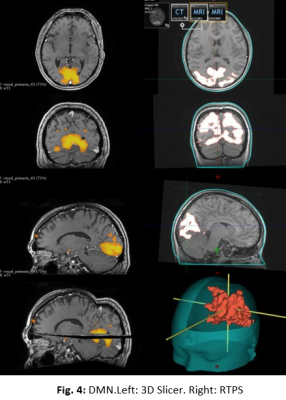
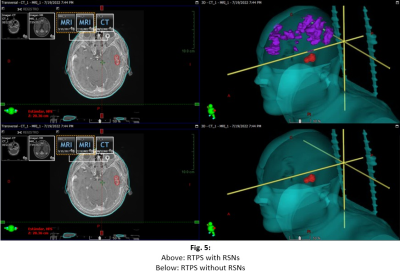
The prospective study was evaluated by the institutional ethics committee. An MR protocol that included a 3DGRE-T1 and an rs-fMRI sequence (Fig. 1) was performed in 4 pathological cases.The analysis of RSNs was performed with FSL, starting with a pre-processing step, where the motion artifacts (FLIRT) and low-frequency deviations are corrected. Subsequently, a two-dimensional independent component analysis (ICA) was performed with the MELODIC function (Z-score threshold of 4). Components associated with the same network (selected using the AI FIX toolbox) were added and normalized to SRS planning, after verifying their morphological localization, temporal signals and frequency spectrums (Fig. 2). The FIX threshold acceptance levels were between 20 and 60, using the default training data, and the configuration was based in Octave and R libraries.Conversion from Nifti to DICOM has been little studied, so the available solutions have some drawbacks in DICOM files resulting in issues with display, data integrity, and incompatibility to be recognized by the planner. To do this conversion we implemented two different tools: Export to DICOM from 3dSlicer and CLI from XMedCon software (Fig. 3). For these problems we develop an in-house Python algorithm (Pydicom and Nibabel) that converts Nifti volumes into DICOM series was developed to incorporate the RSNs into the RTPS (Fig. 4)Two planifications were designed for each subject, with and without RSNs (Fig. 5); clinical visualizations and the segmentation parameters were defined with 3D-Slicer. The planning process was implemented with the Eclipse RTPS and the SRS was carried out using the Halcyon system. Through the dose-volume histogram (DVH), dose homogeneity (HI) and conformity indexes (CI) the dose reduction was evaluated, verifying the absence of hot spots.
Results
In the SRS planning with RSNs segmented as functional OAR (fOAR), the mean dose reduction was between 15 and 20% at fOAR close to PTV. There were no significant changes in the dose received by conventional OAR (SRS planning without fOAR).The implementation of FIX reduced by 64% the classification time of ICA components. The RSNs found in all the patients were: default mode, sensorimotor, frontoparietal and visual networks, prioritizing the conservation of this last network. In three subjects the orbitofrontal network was found, and in only one patient the language, auditory and salience networks were found.3DSlicer and XMedCon present significant drawback, the process rounds to integers the values that were initially in decimals, as a result we can see a pixelated image with noticeable differences to the original Nifti. Another drawback of this tool is the orientation and position of the original volume, this problem does not allow merging directly with its corresponding anatomical image weighted in wT1, requiring an additional transformation to achieve fusion (Fig.3).Conclusion
We emphasize the importance of continuing the few studies on MRIgRT using rs-fMRI (ICA) given the nature of the information provided by this method compared to other tomographic techniques and, thus, reducing the probability of cognitive dysfunctions and adverse side effects.In the next step, the project revolves around adding information from the white matter, which has less neuroplasticity compared to cortical areas. Currently, this information can be obtained through the segmentation with Freesurfer from volumetric wT1, and with tractography it is possible to delimit the tracts associated with the different neural networks. The projection of these tracts on RTPS is an open issue due to their vector nature, which is complex to link with the DICOM protocol.Forming an interdisciplinary group to protocolize both the identification of functional networks (RSNs and tractography) and dose limits becomes a primary need for advancing in this line of MRIgRT research.Discussion
The FIX toolkit improves the identification of RSNs in significantly less time than the traditional method, but still requires expert supervision in the identification of fOAR. It was possible to incorporate the RSNs to the RTPS without modifying the prescribed dose to the target, showing the feasibility of incorporating this information in RT planning. The protocolization of recommended dose limits for the different cortical and subcortical areas is still an open field.Acknowledgements
No acknowledgement found.References
1. M. A. Hayat. Brain Metastases from Primary Tumors, Volume 3. 04 2016.
2. Lu Sun, Baolin Qu, Jinyuan Wang, Zhongjian Ju, Zizhong Zhang, Zhiqiang Cui, Yang Jack, Zhipei Ling, Xinguang Yu, and Longsheng Pan. Integration of functional MRI and white matter tractography in CyberKnife radiosurgery. Technology in Cancer Research & Treatment, 16(6):850–856, April 2017
3. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nature Reviews Neurology. 2017 Jan;13(1):52.
4. McDuff SG, Taich ZJ, Lawson JD, Sanghvi P, Wong ET, Barker FG, Hochberg FH, Loeffler JS, Warnke PC, Murphy KT, Mundt AJ. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. Journal of Neurology, Neurosurgery & Psychiatry. 2013 Dec 1;84(12):1384-91.