3545
Assessing the effects of hyperpolarized [2-13C]lactate saturation on detectable brain metabolism1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Gray Matter, Hyperpolarized MR (Non-Gas), metabolism
Following hyperpolarized [1-13C]pyruvate MRI studies that demonstrated insights into brain metabolism using saturation experiments, this study sought to assess whether metabolic pathways probed by [2-13C]pyruvate could similarly be interrogated. Our investigation showed that not exciting [2-13C]lactate led to a 2-fold increase in [5-13C]glutamate signal, which may indicate compartmentalized metabolism in the brain.Introduction
Recent hyperpolarized carbon-13 (HP-13C) MRI studies in animals1 and humans2 have challenged conventional mechanistic descriptions of brain metabolism. Rather than demonstrating that [1-13C]pyruvate undergoes terminal conversion to [1-13C]lactate and bicarbonate through mutually exclusive pathways, saturation experiments have provided evidence of an interdependent relationship between bicarbonate signal and [1-13C]lactate excitation. Such findings lend support to compartmentalized models of metabolism, wherein glial cells supply lactate as an energy source for neurons3. In this study, we aimed to further interrogate brain metabolism in a healthy volunteer using a HP [2-13C]pyruvate tracer that distinctly reports on Krebs cycle4 activity via [5-13C]glutamate. By variably exciting the [2-13C]lactate resonance and monitoring [5-13C]glutamate signal, it was possible to assess pathway interdependence.Methods
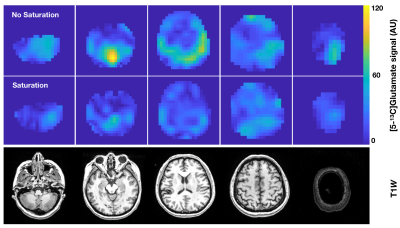
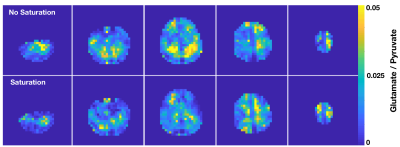
Saturation experiments. A healthy female volunteer who consented to 2 injections of HP [2-13C]pyruvate tracer was recruited to the study. Following polarization on a SPINlab system (GE Healthcare, Waukesha, WI) according to previously described methods5, [2-13C]pyruvate (0.43 mL/kg) was intravenously administered as an aqueous solution at 5 mL/s. HP data were acquired on a 3T MR750 scanner (GE Healthcare) equipped with a dual-tuned 13C/1H 24/8-channel receiver coil (Rapid Biomedical, Munich, Germany) using a dynamic multi-resolution echo-planar imaging (EPI) sequence6 that featured spectral-spatial excitation (spatial resolution: Pyr/Lac/Glu = 8x8/24x24/24x24 mm2 in-plane, 3-cm slice thickness; TR/TE = 100 ms/25.1 ms, 240×240 mm2 FOV, 5 slices, 20 timepoints, 3s temporal resolution, 60s total acquisition time). The center frequency of [2-13C]pyruvate was determined in relation to water and [1-13C]pyruvate: f0-C2pyr = f0-H20*C + 1100 Hz, where C is an empirical gyromagnetic conversion factor (0.251491899). To assess the effects of [2-13C]lactate excitation, two flip schemes were employed: one without lactate excitation (αPyr/ αGlu /αLac,downfield/ αLac,upfield = 200/600/00/00) and one with lactate saturation at (αPyr/ αGlu /αLac,downfield/ αLac,upfield/ = 200/600/900/900), with a 15 min. separation between the two injections.HP-13C Post-processing. Muti-resolution dynamic EPI data were prewhitened via Cholesky decompostion7, channel-combined using complex weights from the fully sampled pyruvate signal8, phased and denoised9. Lactate and glutamate images were then interpolated to the higher pyruvate resolution. A comparative analysis of glutamate-to-pyruvate was performed between the saturated versus unsaturated lactate flip schemes.
Results/Discussion
Figure 1 demonstrates the increased level of [5-13C]glutamate with the unsaturated versus saturated lactate flip scheme in the healthy volunteer, with the time-to-injection being 56 s and 53 s, respectively. This translated into an average factor increase in [5-13C]glutamate signal of 2.0. The ratio of [5-13C]glutamate / [2-13C]pyruvate is also shown to be elevated in the unsaturated case. These data collectively demonstrate some level of glutamate and lactate pathway coupling for C2-labeled HP metabolism in the brain, which is suggestive of compartmentalized models hypothesized by prior groups performing saturation experiments with C1-labeled pyruvate.Acknowledgements
This study was supported by NIH P01CA118816 and NIH R01CA273028-01.References
1. Bogh N, Grist JT, Rasmussen CW, Bertelsen LB, Hansen ESS, Blincher JU, et al. Lactate saturation limits bicarbonate detection in hyperpolarized 13C-pyruvate MRI of the brain. Magn. Reson. Med. 2022;88(3):1170-11792.
2. Uthayakumar B, Bragagnolo N, Soliman H, Chen AP, Endre R, Perks WJ, et al. Evidence of lactate shuttling in the human brain with hyperpolarized 13C-MRI. Proc. Int. Soc. Magn. Reson. Med. 2022;Abstract#10673.
3. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994;91:10625-10629.
4. Martínez-Reyes, I., Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11,102.5.
5. Chung BT, Chen HY, Gordon J, et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J Magn Reson. 2019; 309:106617.
6. Gordon JW, Autry AW, Tang S, et al. A variable resolution approach for improved acquisition of hyperpolarized 13C metabolic MRI. Magn Reson Med. 2020;84(6):2943-2952.
7. Hansen MS. Parallel Imaging Reconstruction I: Cartesian. Proc. Intl. Soc. Mag. Reson. Med. 18 (2010)
8. Zhu Z, Zhu X, Ohliger M, Cao P, et al. Coil combination methods for multi-channel hyperpolarized 13C imaging data from human studies. JMR 2019;301:73-39.9.
9. Kim Y, Chen HY, Autry AW, et al. Denoising of hyperpolarized 13C MR images of the human brain using patch-based higher-order singular value decomposition. Magn. Reson. Med. 2021;86:2497-2511.