3539
Reproducibility of Diffusion Microstructures Over Different Diffusion Gradient Directionalities1Symbiosis Centre for Medical Image Analysis, Symbiosis International (Deemed University), Pune, India, 2Department of Radiology, National Institutes of Mental Health and Neuroscience, Bengaluru, India, 3National Institutes of Mental Health and Neuroscience, Bengaluru, India
Synopsis
Keywords: Neuroinflammation, Brain, Diffusion Imaging, freewater, reliability
Medical imaging can offer several clinically valuable biomarkers which require accuracy across the acquiring technologies. Our study investigated the variability and reliability of diffusion microstructures over different diffusion gradient direction schemes, including high angular resolution diffusion imaging sequences and a parallel slice acquisition technique. The study's results demonstrated the 20-directional parallel slice acquisition clinically feasible sequence for standard DTI analysis with less variability and more reliability in matrices, while multishell sequences are more suitable for microstructural and tractography analysis.Introduction
Medical imaging can offer several clinically valuable biomarkers; however, the accuracy of its metrics must first be established on a broad scale and include the variety of scanning technology and software used to obtain the data.1-3 DTI investigations of patients reveal abnormalities in the microstructure of white matter in several pathways across a spectrum of neurological diseases4. In this work, the variability of DTI matrices across varying numbers of diffusion gradient directions was evaluated in order to identify the clinically best possible diffusion sequence.Method
In this experiment, we scanned five healthy participants using six distinct DWI sequence protocols on the Siemens Prisma to gain a better understanding of the variation in diffusion microstructures resulting from the varying numbers of diffusion gradient directions. These sequences had TR/TE = 8600/82 ms, voxel size of 1 mm isotropic, and numbers of diffusion gradient directions of 1) 6 gradient directions (6d); 2) 20 gradient directions (20d); 3) 20 gradient directions with simultaneous multi-slice (SMS) factor 2 (20dsms); 4) 30 gradient directions (30d), and 5) 64 gradient directions (64d) with a b-value of 1000s/mm2. The sixth protocol is a multishell 90 diffusion gradient directions (MS) acquisition with TR/TE = 4200/91 ms, SMS = 2, and b-values of 500, 1000, and 2500 s/mm2 (30 directions per b-value). All the dMRI data were preprocessed using FSL v6.0.5 (https://fsl.fmrib.ox.ac.uk/), which includes a manual quality check followed by distortion correction using reverse phase encoding directional scan in the top-up function and eddy noise and motion correction by registering with the distortion-corrected B0 image non-linearly. Following correction, non-brain tissues were excluded using the brain extraction tool, and fractional anisotropy (FA) and other diffusivity maps, including mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), were calculated. The bi-tensor model was used, which divides each voxel into two compartments—an intracellular tissue compartment and an extracellular water compartment to compute extracellular isotropic microstructures such as free-water (FW) and corrected diffusivity maps (FAt and MDt). All processed images were subjected to statistical analysis, with means and variances calculated for brain tissue subtypes (gray matter (GM), white matter (WM), and corticospinal fluid (CSF)) and WM regions of interest (RoIs). The repeatability of diffusion microstructures was evaluated with the reproducibility factor coefficient of variation (CoV)5.Results
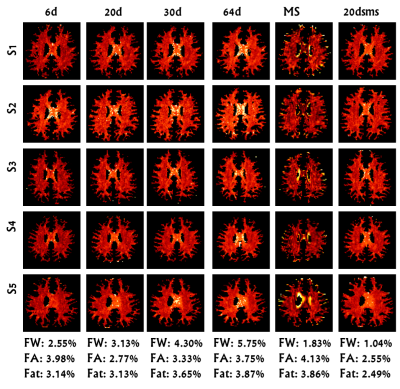
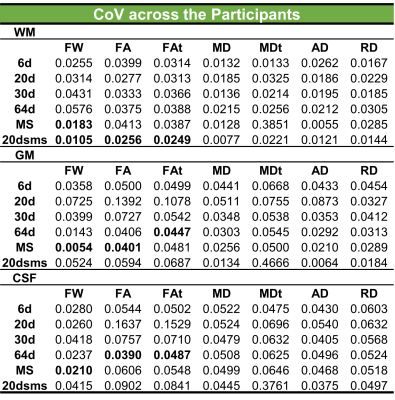
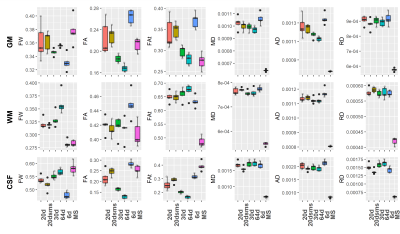
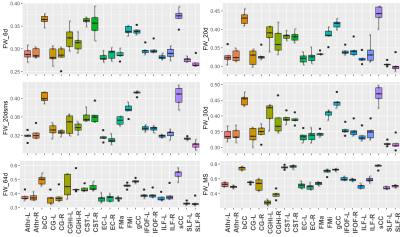
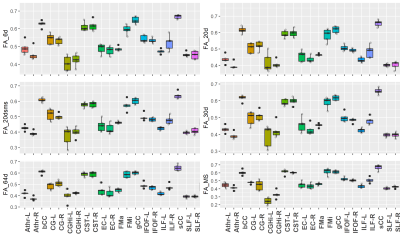
Figure 1 displays representative FW maps of the WM tissue type as well as the coefficients of variation (CoVs) of FW, FA, and corrected FAt across participants and sequences. The maps visually represented the repeatability of FW across sequences for each participant. Figure 2 shows a box plot representation of the mean distributions of all diffusion microstructures for all six protocol sequences over participants for brain tissue types, and table 1 shows the variability scores of all diffusion microstructures. In this study, the variability of FW, FA, and FAt of the 20dsms sequence for WM tissues was the lowest compared to other sequences, at 1.05%, 2.56%, and 2.49%, respectively. While for GM tissues, the CoVs of FW and FA were 0.54% and 4.01% for MS sequence protocol and 4.45% for FAt in 64d sequence protocol. For CSF tissues, the FW CoV for MS was 2.1%, while the CoVs of FA and FAt for 64d sequence protocol were 3.9% and 4.87 %, respectively. Figure 3 shows the FW mean distribution of WM RoIs such as body of corpus collosum (bCC), genu of corpus collosum (gCC), splenuim of corpus collosum (sCC), external capsule (EC), and WM tracts such as anterior thalamic radiation (AThr), cingulum (CG), corticospinal tract (CST), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), and forceps minor and major (FMi, FMa) while mean FA distribution were shown in figure 4.Conclusion
In this study, results demonstrated that the 20 gradient direction with the SMS sequence gave less variability for WM tissues, while HARDI sequences such as 64 directional and MS gave more reliability for GM and CSF. Similarly, for the WM ROIs level, MS illustrated higher reliability in FW estimates.Our results indicate that 20 directions with an SMS sequence is clinically optimally feasible for standard diffusion markers based on the dependability of microstructures and requires less scanning time than HARDI sequences. MS sequence is better suited for tractography and extracellular microstructure-based markers due to its higher sensitivity and lower gradient value for extracellular water.Acknowledgements
We would like to acknowledge that this study was a part of work for SERB EMR/2017/004523 funded by SERB, DST, India, with Dr. Madhura Ingalhalikar (Co-PI) and Dr Jitender Saini, (PI) NIMHANS, BangaloreReferences
1. Coelho, S., Baete, S.H., Lemberskiy, G., Ades-Aron, B., Barrol, G., Veraart, J., Novikov, D.S. and Fieremans, E., 2022. Reproducibility of the Standard Model of diffusion in white matter on clinical MRI systems. NeuroImage, 257, p.119290.
2. Irfanoglu, M.O., Sadeghi, N., Sarlls, J. and Pierpaoli, C., 2021. Improved reproducibility of diffusion MRI of the human brain with a four‐way blip‐up and down phase‐encoding acquisition approach. Magnetic resonance in medicine, 85(5), pp.2696-2708.
3. Niogi, S.N., Mukherjee, P., Ghajar, J., Johnson, C., Kolster, R.A., Sarkar, R., Lee, H., Meeker, M., Zimmerman, R.D., Manley, G.T. and McCandliss, B.D., 2008. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. American Journal of Neuroradiology, 29(5), pp.967-973.
4. Mukherjee, P., Chung, S.W., Berman, J.I., Hess, C.P. and Henry, R.G., 2008. Diffusion tensor MR imaging and fiber tractography: technical considerations. American Journal of Neuroradiology, 29(5), pp.843-852.
5. Palacios, E.M., Martin, A.J., Boss, M.A., Ezekiel, F., Chang, Y.S., Yuh, E.L., Vassar, M.J., Schnyer, D.M., MacDonald, C.L., Crawford, K.L. and Irimia, A., 2017. Toward precision and reproducibility of diffusion tensor imaging: a multicenter diffusion phantom and traveling volunteer study. American Journal of Neuroradiology, 38(3), pp.537-545.
Figures