3532
Quantitative susceptibility source separation improves the reader agreement in the identification of MS paramagnetic rim lesions1Department of Neurology, Weill Cornell Medicine, New York City, NY, United States, 2Department of Radiology, Weill Cornell Medicine, New York City, NY, United States
Synopsis
Keywords: Multiple Sclerosis, Neuroscience
Multiple Sclerosis (MS) is an autoimmune disorder characterized by focal inflammatory demyelination. Paramagnetic rim lesions (PRLs) form an important subset of chronic active MS lesions that show a hypocellular core and persistent glial inflammation and continuing myelin damage at the rim on histopathology. PRLs are a critical clinical target for MS therapy. Our goal in this project is to improve overall detection of PRLs through utilization of quantitative paramagnetic source maps.Introduction
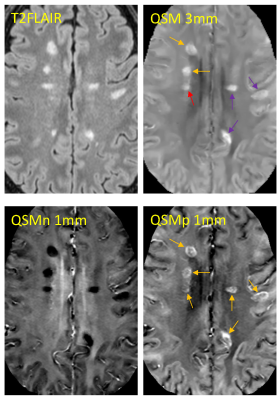
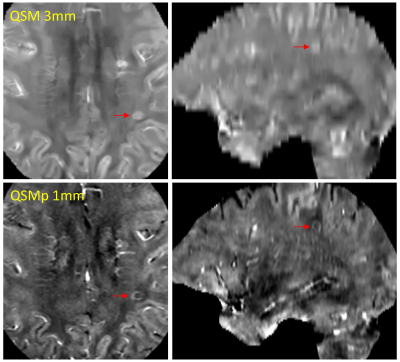
Paramagnetic rim lesions (PRLs) in multiple sclerosis (MS) are characterized by the presence of iron-laden activated microglia on histology1,2 and a hyperintense rim appearance on quantitative susceptibility mapping (QSM)2 or a dark rim on the high-pass filtered phase image3. These lesions tend to show larger tissue damage compared to those without rim4 and are associated with worse clinical outcome5,6. However, their detection using current clinical QSM protocol7 can be challenging partly due to the relatively thick 3mm slices and partly because both myelin loss and iron accumulation, two pathological processes often coexisting in the lesion rim area, can give rise to high susceptibility signal on QSM. This confounding effect can lead to suboptimal reader agreement even among experts8. The objective of this study was to improve PRL detection and inter-reader reproducibility by optimizing a clinically translatable QSM acquisition with 1mm slices and applying a recently developed quantitative susceptibility source separation method9 which can resolve positive (iron) and negative (myelin) sources without requiring additional scan time.Purpose
To compare reader agreement and difficulty of rim lesion detection on 3MM QSM and 1MM paramagnetic susceptibility source images.Methods
The patient cohort was composed of twenty-five multiple sclerosis patients. Each subject was scanned on a clinical 3T MRI scanner (Magnetom Skyra, Siemens, Erlangen, Germany) using a 20-channel head/neck coil. For the purpose of susceptibility mapping, the imaging protocol included two 3D mGRE acquisitions with the following parameters: a) FOV=25.6 cm, TE1/dTE= 6.28/4.06 ms, #TE=10, TR=48 ms, rBW = 260 Hz/px, FA=15, voxel size=0.8*0.8*3 mm, slice resolution 100%, acquisition time 4 min 10 sec; b) FOV=24.0 cm, TE1/dTE= 6.28/4.06 ms, #TE=8, TR=40 ms, rBW = 260 Hz/px, FA=15, voxel size=0.4*0.4*1 mm, slice resolution 50%, acquisition time 5 min 5 sec. QSM was reconstructed from complex GRE images using morphology-enabled dipole inversion method with global CSF referencing (MEDI+0)10. Susceptibility source separation for separate quantification of myelin and iron contributions was performed using R2*QSM9.Two readers independently classified each lesion as PRL or rim negative on 3MM QSM and 1MM QSMp. Kappa statistics were used to compare reader agreement. For each rim classification, the reader determined the difficulty of the rim detection on a binary scale. Chi-squared analysis was used to test the significance of the difference in proportions of the PRLs classified as "difficult” between the 3MM QSM and the 1MM QSMp for each reader.
Results
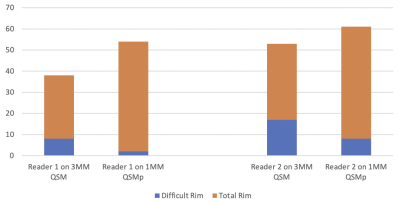
A total of 824 MS lesions were identified in the patient cohort. There was moderate agreement (95.4% agreement, Cohen's k = 0.56) on the 3MM QSM reading while there was substantial agreement on the 1MM QSMp reading (96.1%, Cohen’s k = 0.69). There was a higher level of agreeability between the readers on 1MM QSMp which can be seen in Figure 1.The difficulty of rim detection was also considered by both readers independently. For reader 1, the proportion of difficult rim lesions on 3MM QSM and 1MM QSMp was 8/30 and 2/52, respectively (Figure 2). For reader 2, the proportion of difficult rim lesions on 3MM QSM and 1MM QSMp was 17/36 and 8/61, respectively (Figure 2). For readers 1 and 2, the proportion of rims that were classified as difficult on 1MM QSMp was significantly lower than on 3MM QSM (χ2 = 9.25, p = 0.002; χ2 = 5.96, p = 0.015); moreover, rim detection was easier on 1MM QSMp.
Conclusion
We found that rim lesions were easily identified on 1MM QSMp. Our results support further investigation and use of QSMp to detect PRLs in MS patients.Acknowledgements
No acknowledgement found.References
1. Hametner S, Wimmer I, Haider L, Pfeifenbring S, Bruck W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013;74:848-61.
2. Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol 2021;8:877-86.
3. Absinta M, Sati P, Gaitan MI, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol 2013;74:669-78
4. Yao Y, Nguyen TD, Pandya S, et al. Combining quantitative susceptibility mapping with automatic zero reference (QSM0) and myelin water fraction imaging to quantify iron-related myelin damage in chronic active MS lesions. AJNR Am J Neuroradiol 2018;39:303-10.
5. Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 2019;76:1474-83.
6. Huang W, Sweeney EM, Kaunzner UW, Wang Y, Gauthier SA, Nguyen TD. Quantitative susceptibility mapping versus phase imaging to identify multiple sclerosis iron rim lesions with demyelination. J Neuroimaging. 2022 Jul;32(4):667-675.
7. Zhang S, Liu Z, Nguyen TD, Yao Y, Gillen KM, Spincemaille P, Kovanlikaya I, Gupta A, Wang Y. Clinical feasibility of brain quantitative susceptibility mapping. Magn Reson Imaging. 2019 Jul;60:44-51.
8. Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol 2018;39:1233-8.
9. Dimov, A. V., T. D. Nguyen, K. M. Gillen, M. Marcille, P. Spincemaille, D. Pitt, S. A. Gauthier, and Y. Wang. "Susceptibility Source Separation from Gradient Echo Data Using Magnitude Decay Modeling." J Neuroimaging (2022).
10. Dimov, A. V., T. D. Nguyen, P. Spincemaille, E. M. Sweeney, N. Zinger, I. Kovanlikaya, B. H. Kopell, S. A. Gauthier, and Y. Wang. "Global Cerebrospinal Fluid as a Zero-Reference Regularization for Brain Quantitative Susceptibility Mapping." J Neuroimaging (2021).
Figures