3528
Inhibition of phospholipase A2 by mepacrine reduces total choline in a rat model of Alzheimer’s disease1Sunnybrook Research Institute, Toronto, ON, Canada, 2Synaptive Medical, Toronto, ON, Canada, 3Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Alzheimer's Disease, Preclinical, neuronal loss
We examined the effect of the PLA2 inhibitor mepacrine on the levels of choline and other brain metabolites in the TgF344-AD rat model of Alzheimer's disease. Previous studies have demonstrated elevated levels of brain cholines in this model, similar to those observed in humans and attributed to increased membrane turnover. Total choline was higher in transgenic animals at baseline, but decreased following mepacrine treatment. Total choline also decreased in wildtype animals following treatment, consistent with the hypothesis that mepacrine reduces PLA2-mediated membrane turnover. Based on this finding, MRS choline measures may also serve as a biomarker for PLA2-mediated inflammatory processes.Introduction
The pathology of Alzheimer’s disease (AD) has several key hallmarks, including the accumulation of amyloid plaques and neurofibrillary tangles in the brain, neuroinflammation and neuronal loss, eventually leading to cognitive impairment. Magnetic resonance spectroscopy (MRS) studies have demonstrated elevated levels of brain cholines in humans with AD1 and animal models2, attributed to increased membrane turnover. More specifically, it has been hypothesised that AD pathology results in increased activity of phospholipase A2 (PLA2) which breaks down membrane-bound phosphatidylcholine, resulting in the release of glycerophosphocholine (GPC) and phosphocholine (PCh), along with arachidonic acid (AA). Therefore, MRS measures of total choline levels (GPC+PCh) may serve as a useful biomarker of PLA2 activity, and the downstream inflammatory pathology initiated by AA in AD.In this study, we examine the effect of the PLA2 inhibitor mepacrine on the levels of choline and other brain metabolites in the TgF344-AD rat model3. This model exhibits amyloid plaques and tau pathology, as well as neuronal loss and cognitive impairment, and has been previously shown to have elevated brain choline levels. We hypothesize that inhibition of PLA2 with mepacrine will result in a decrease in total choline (tCho) levels observed by MRS.
Methods
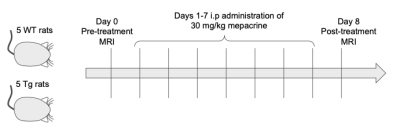
Eleven Fischer 344 rats - 5 wildtype (WT; 3F, 2M), 6 transgenic (TgF344-AD, 6F) - aged 13 months were studied. All animals underwent a baseline MRS scan, followed by treatment with 30 mg/kg mepacrine dissolved in PBS, administered intraperitoneally each day for 7 days (Figure 1). Animals then underwent a post-treatment scan on day 8. One animal died before post-treatment MRS and was excluded from analysis.MRI was conducted under 1-4% isoflurane on a 7 T Bruker Avance BioSpec (Bruker BioSpin, Billerica, MA, USA) with an 86 mm birdcage coil for RF transmit and a four-channel rat brain array for receive. Anatomical T2-weighted RARE images were acquired for MRS voxel placement (Coronal: TE=9.17 ms, TR=2500 ms, 4 averages, 0.234 x 0.234 x 0.7 mm3 resolution. Axial: TE=9.17 ms, TR=6282 ms, 4 averages, 0.234 x 0.234 x 0.5 mm3 resolution).
For MRS, a 3.5 x 3.5 x 2 mm3 voxel was placed in the left dorsal hippocampus (Figure 2) and a localized shim was performed using the Mapshim method (ParaVision 6.0.1, Bruker) to get a linewidth <10 Hz. MRS data were acquired with a Point RESolved Spectroscopy sequence with water suppression (TE=8.77 ms, TR=2500 ms, 2048 data points, spectral width 4000 Hz, 250 averages).
Spectra were analyzed using LCModel (Stephen Provencher Inc., Oakville, ON, Canada). The basis set contained 18 simulated metabolites and 9 macromolecule basis functions developed previously4. Quantification was performed using the unsuppressed water signal for reference.
A decrease in choline levels between pre- and post-mepacrine treatment groups for each genotype was tested using a one-sided t-test, as was the increase between wildtype and TgF344-AD groups at each timepoint. All other metabolite comparisons used two-sided t-tests.
Results
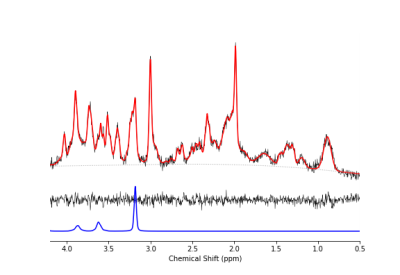
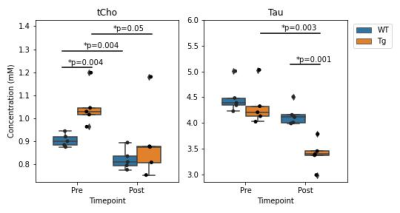
Figure 3 shows a representative dataset along with the LCModel fit for one of the wildtype animals prior to treatment. The average linewidth of the water reference peak was 9.2 +/- 0.4 and the average SNR of the NAA peak was 15 +/- 3.Figure 4 shows the concentrations of total Choline (tCho) and Taurine (Tau) for animals in each group before and after treatment with mepacrine. tCho was higher in transgenic animals at baseline, but decreased following mepacrine treatment. Wildtype animals also showed a decrease in tCho following treatment. The only other metabolite that showed a significant change with treatment was taurine, which was not significantly different at baseline, but decreased in TgF344-AD animals following mepacrine treatment.
Discussion
Pre-treatment, the elevated brain tCho levels in the TgF344-AD rat model relative to wildtype at 13 months of age is consistent with previous work in this model2 and human studies1. Although myo-inositol (Ins) has previously been shown to be significantly elevated in TgF344-AD rats at 16 months of age, we did not detect elevated Ins levels, possibly due to the small sample size or the younger age of the rats in this study. The significant decrease in choline levels following mepacrine treatment is consistent with the hypothesis that mepacrine reduces PLA2-mediated membrane turnover and reduces free cholines. Based on this finding, MRS choline measures may also serve as an important biomarker for PLA2-mediated inflammatory processes.Mepacrine treatment also resulted in a significant reduction in Taurine levels (Figure 4b) but the physiological interpretation remains unclear at this time.
This study is limited by the lack of a control group that did not receive mepacrine treatment. Moreover, the study lacks an analysis of the effects of Mepacrine treatment on cognitive outcomes. Future work will include appropriate control groups as well as behavioural testing to assess the effects of mepacrine treatment on learning and memory function. Future work will also use ex-vivo histological analysis to quantify neurons, glial cells and inflammation.
In conclusion, MRS measures of tCho demonstrate promise as a translational biomarker of PLA2 activity in the brain. Moreover, these findings motivate further investigation into the use of PLA2 inhibitors to target neuroinflammatory pathways associated with AD pathophysiology.
Acknowledgements
Ved Hatolkar was responsible for data acquisition and contributed equally to this work as co-first author. He was funded by the Hurvitz Brain Sciences Summer Student Research ProgramReferences
1. Marjańska M, McCarten JR, Hodges JS, Hemmy LS, Terpstra M. Distinctive Neurochemistry in Alzheimer's Disease via 7 T In Vivo Magnetic Resonance Spectroscopy. J Alzheimers Dis. 2019; 68(2): 559-569.
2. Fowler CF, Goerzen D, Devenyi GA, Madularu D, Chakravarty MM, Near J. Neurochemical and cognitive changes precede structural abnormalities in the TgF344-AD rat model. Brain Commun. 2022; 4: fcac072.
3. Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J Neurosci. 2013 Apr 10;33(15): 6245-56.
4. Fowler CF, Madularu D, Dehghani M, Devenyi GA, Near J. Longitudinal quantification of metabolites and macromolecules reveals age- and sex-related changes in the healthy Fischer 344 rat brain. Neurobiol Aging. 2020; 101: 109-122.
Figures