3523
Dynamic Changes in Quantitative MRI-based Biomarkers of Alzheimer’s Disease and Its Association with Cognitive Decline – A Longitudinal Study1Peking University Shenzhen Hospital, Shenzhen, China, 2The Chinese University of Hong Kong, Hong Kong, SAR, China
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease
Alzheimer's disease (AD) resemblance atrophy index (AD-RAI) is a novel MRI-based machine-learning derived imaging biomarker for AD that is valid in detection of early AD. We selected 318 CU and MCI subjects in ADNI with four-year follow-up with 1471 serial MRI scans to assess longitudinal changes of the MRI biomarkers (i.e., AD-RAI, HV, HF, BPV, BPF) in correlation with the change in time and conversion status. We used generalized linear mixed-effects models to compute and show the predictive value. AD-RAI over time showed the highest classification accuracy for predicting converters when compared with other imaging biomarkers.Introduction
Developing a biomarker of Alzheimer’s Disease (AD) that can be used to monitor disease progression or to serve as a surrogate outcome measure in clinical trials is important in the management and research of AD. Changes in such biomarkers over time should correlate with changes in cognitive function. Although serial measurements of biomarkers based on CSF and PET may be used to monitor disease progression.1 CSF is invasive while PET is associated with radiation exposure, and both are relatively expensive. Previous studies have commonly utilized change in hippocampal volume (HV) as a surrogate marker for monitoring disease progression.2,3 However, apart from HV, progressive atrophy of other cognitive relevant brain regions is also present.4 AD-RAI is an MRI-based machine learning-derived composite index reflecting multi-region atrophy severity of AD. Previous studies have shown that it is clinically valid in diagnosing AD at the MCI and dementia stage.5 In this study, we aimed to investigate the association between the serial change of AD-RAI and cognitive progression overtime among CU and MCI subjects and to compare it with other conventional MRI biomarkers (i.e., HV, hippocampal fraction (HF), brain parenchymal volume (BPV) and fraction (BPF)), which have been used in the past as surrogate markers for AD.Methods
We selected eligible subjects from the Alzheimer’s Disease Neuroimaging Initiative [ADNI] (http://adni-info.org) databases (n=1740) and included those aged 55 to 90 years, diagnosed with CU and MCI at baseline with high-quality volumetric MRI data of T1W scans with 4-year follow-up and excluded those whose diagnoses have reversion or poor image quality at baseline or at any time-point in the 4-year follow-up. We defined converters as those who had significant clinical cognitive progression in the 4-year follow-up (i.e., CU to MCI, or MCI to AD dementia), while non-converters were those who remained stable in the 4-year follow-up. A total of 318 subjects, including 203 non-converters and 115 converters, were recruited with 1471 serial MRI scans in total. We also considered the influences from age at baseline, gender, education, and Apolipoprotein E ε4 allele (APOE4) genotypes. All 3D T1W images were analyzed using the AccuBrain software system, which is an automated computational tool that can be adapted to a cloud platform available for research use, where a report consisting of AD-RAI and volumetric measures of multiple brain regions can be generated automatically back to the end-users within 10 minutes. In this study following data were extracted for further analysis: AD-RAI, HV, HF, BPV, and BPF. We used a linear mixed-effects model (LMM) and generalized linear mixed-effects models (GLMM) in SPSS 26.0 to explore the serial changes of the imaging biomarkers and interaction effects over time and to compute the accuracy of classification.Results
LMM showed that AD-RAI of converters increased significantly faster than non-converter over time (P < .001), and HV, HF, and BPF of converters decreased significantly faster than non-converters over time (P < .001, P < .001, P = .004, respectively). Besides, the annualized hippocampal atrophy rate was 1.2% for non-converter and 3.3% for converters when using the corresponding HV at baseline as the reference. Fig. 1-5 showed the means with error bars of 95% CI of each biomarker. In GLMM, we further found that dynamic AD-RAI over time had the highest classification accuracy (74.2%) for predicting converters when compared with other imaging biomarkers (≤ 70.2%).Discussion
Our mean finding that serial change in AD-RAI outperformed that of hippocampal and global brain measures in correlating with cognitive progression shows that serial change of a biomarker that can capture the multi-brain regional atrophy characteristic to AD is better than that of a single region in correlating with cognitive progression. Our secondary finding that the changing rate of HV and HF was faster in converters than in non-converters was in agreement with previous studies.2-4,6,7 We also subsequently found that the rate of changes of hippocampal measures had a better correlation with that of whole-brain measures, which was also consistent with results from some previous studies.2 Moreover, AD-RAI outperformed hippocampal and global brain measures showing the change in AD atrophy pattern matters. Noteworthy is that the superiority of the association between the change of AD-RAI with cognitive progression over other imaging measures was present even after adjusting to age, gender, education, and APOE4. AD-RAI can predict the risky subjects at the individual level before clinical progression and help to select the risky participants in clinical trials.Conclusion
Serial AD-RAI outperforms other conventional biomarkers in correlating with cognitive progression. It may be used as a surrogate marker for monitoring disease progression or treatment response in AD.Acknowledgements
We would like to thank Lei Zhao and Yishan Luo for their help in the data processing. We are also grateful for the research volunteers, their families, and the investigators at the ADNI databases.References
1. Jack CR, Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. Feb 2013;12(2):207-16.2. den Heijer T, van der Lijn F, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. Apr 2010;133(Pt 4):1163-72.
3. Franko E, Joly O, Alzheimer's Disease Neuroimaging I. Evaluating Alzheimer's disease progression using rate of regional hippocampal atrophy. PLoS One. 2013;8(8):e71354.
4. Jack CR, Jr., Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. Feb 24 2004;62(4):591-600.
5. Zhao L, Luo Y, Lew D, et al. Risk estimation before progression to mild cognitive impairment and Alzheimer's disease: an AD resemblance atrophy index. Aging (Albany NY). Aug 29 2019;11(16):6217-6236.
6. Nadal L, Coupe P, Helmer C, et al. Differential annualized rates of hippocampal subfields atrophy in aging and future Alzheimer's clinical syndrome. Neurobiol Aging. Jun 2020;90:75-83.
7. Rana AK, Sandu AL, Robertson KL, et al. A comparison of measurement methods of hippocampal atrophy rate for predicting Alzheimer's dementia in the Aberdeen Birth Cohort of 1936. Alzheimers Dement (Amst). 2017;6:31-39.
Figures
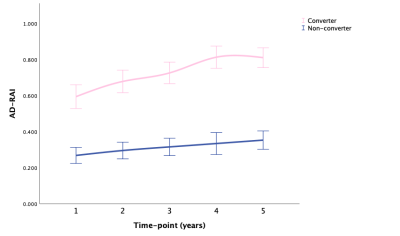
Fig. 1 AD-RAI: means with 95% CI over time in converter and non-converter
Abbreviations: AD-RAI, Alzheimer’s disease resemblance atrophy index; CI, confidence interval.
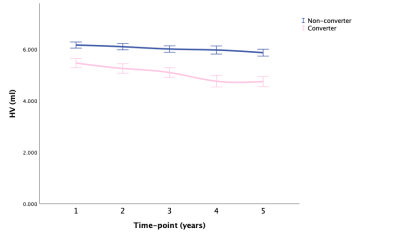
Fig. 2 HV: means with 95% CI over time in converter and non-converter
Abbreviations: HV, hippocampal volume; CI, confidence interval.
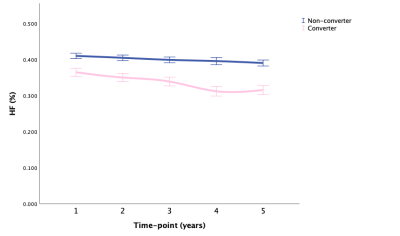
Fig. 3 HF: means with 95% CI over time in converter and non-converter
Abbreviations: HF, hippocampal fraction; CI, confidence interval.
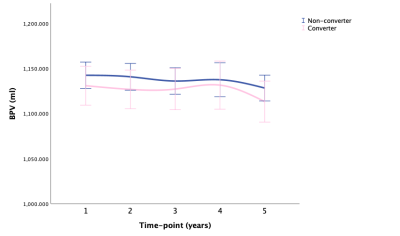
Fig. 4 BPV: means with 95% CI over time in converter and non-converter
Abbreviations: BPV, brain parenchymal volume; CI, confidence interval.
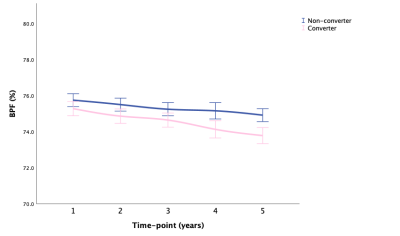
Fig. 5 BPF: means with 95% CI over time in converter and non-converter
Abbreviations: BPF, brain parenchymal fraction; CI, confidence interval.