3519
Fixel based analysis to investigate white matter alterations in individuals with Mild cognitive impairment and Alzheimer’s disease1Department of Neurology, University of California Davis, Davis, CA, United States, 2Department of Biomedical Engineering, University of California Davis, Davis, CA, United States
Synopsis
Keywords: Alzheimer's Disease, Diffusion/other diffusion imaging techniques, Fixel based analysis
We investigated fibre tract-specific changes in the whole-brain white matter in individuals with Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) using Fixel Based Analysis (FBA). The results show significant reduction in microstructural fibre bundles in AD, in regions including splenium of the corpus callosum, fornix, and the uncinate fasciculus. FBA-derived measures, including fibre density (FD), and combination of fibre density and cross-section (FDC) demonstrated sensitivity in detecting microstructural white matter alterations in AD. These findings highlight the utility of FBA as a potential biological marker for providing valuable insights into pathophysiologic changes in AD.Introduction
Alzheimer’s disease (AD) is the leading cause of dementia, characterized by the accumulation of amyloid β plaques and neurofibrillary tangles of hyperphosphorylated tau, and is associated with altered white matter (WM) microstructures 1, 2. However, when and where in the brain white matter alterations present over the course of disease is yet to be fully understood. Over the past decade, diffusion tensor Imaging (DTI) MRI technique has been extensively used to detect WM microstructural changes in AD 1. However, DTI is limited with its ability to model a complex and crossing fibre populations, as a result, DTI based findings are often unreliable and difficult to interpret.Fixel-based analysis (FBA) is a relatively novel technique that addresses the limitations of DTI and enables the investigation of fibre specific WM changes at the microscopic level 3.
FBA analysis is based on the post-processing of DWI data using constrained spherical deconvolution (CSD) technique 4, which allows multiple fibre orientations to be characterized within voxels. In this study, we aimed to investigate the fibre tract-specific WM changes as measures of fibre density (FD), fibre Cross-section (FC), and combination of fibre density and cross-section (FDC). We hypothesised that there would be significant reductions in WM fibre density and cross-section in AD relative to MCI and controls. In this abstract, we report our preliminary FBA findings for tracts of the whole-brain white matter in individuals with AD relative to MCI and controls.
Methods
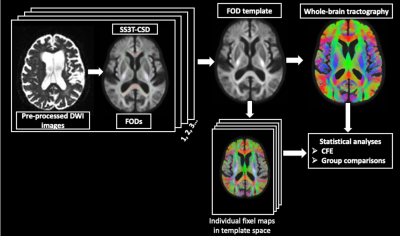
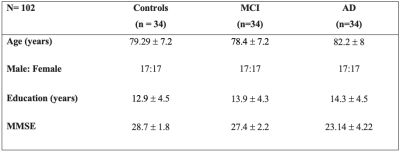
102 participants were included in this study, including 34 individuals with AD, 34 with MCI, and 34 control subjects (Table 1).DWI data were obtained on a 3T Siemens TimTrio scanner (resolution=2mm, 60 directions, at b= 1000s/mm2, and 5 volumes at b=0) and pre-processed using MRtrix3 5. Response functions for single-fibre WM as well as gray matter (GM) and cerebro-spinal fluid (CSF) were estimated using an unsupervised method 6. A group average response function was estimated to obtain Fibre Orientation Distributions (FODs). Single-Shell 3-tissue CSD (SS3T-CSD) was performed to obtain WM-like FODs as well as GM-like and CSF-like compartments in all voxels, using MRtrix3Tissue, a fork of MRtrix3 5. A study-specific unbiased FOD template was generated from 30 individuals (including 10 controls, 10 MCI, and 10 AD), and was used as a reference to register FOD images from all the subjects.
The whole-brain FBA maps, including FD, FC, and FDC, were generated. A whole-brain tractogram, derived from probabilistic tractography, was generated on the FOD template to obtain connectivity information. Statistical analyses were performed using connectivity based fixel enhancement (CFE) at each fixel using general linear model 7. Whole-brain comparisons were performed to identify group differences in fixel measures (Figure 1).
Results
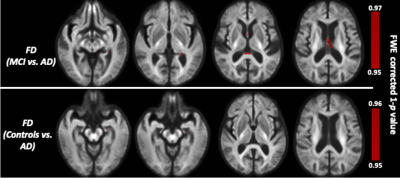
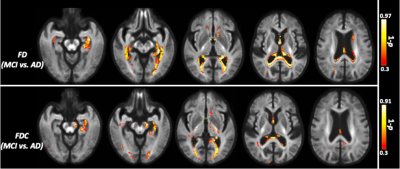
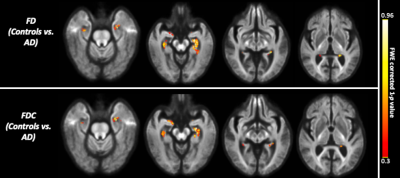
The whole-brain FBA showed significant reduction in FD in individuals with AD compared to MCI, in regions including splenium of corpus collosum, fornix, and the left uncinate fasciculus. Furthermore, AD showed significant reduction in FD in the left uncinate fasciculus compared to controls. Figure 2 shows WM tracts where significant (FWE corrected p <0.05) reductions in FD were observed.We observed subtle reduction in FD and FDC metrics (although the differences were not statistically significant after FWE corrections) in regions including corpus callosum (bilaterally), forceps majors (bilaterally), and uncinate fasciculus in AD compared to MCI. The reduction in FD was more pronounced compared to the reduction in FDC (Figure 3).Furthermore, FD and FDC were observed to be reduced (although not statistically significant after FWE correction) in regions including uncinate and the arcuate fasciculus (bilaterally) in AD relative to controls (Figure 4). We did not observe notable difference in FC in any groups. There were no reductions in FBA measures in MCI relative to controls.Discussion
This study used FBA to investigate fibre tract-specific whole-brain white matter changes in individuals with MCI and AD. The results suggested a trend of WM degeneration in cerebral regions including splenium of corpus callosum, fornix and the left uncinate fasciculus. The fibre pathway connecting such regions has been previously identified to be functionally impaired in AD 8. Our findings are in line with the findings from an earlier study 9. This study has however some limitations. First, DWI data were obtained with lower b- value. Data with b-value higher than 2000 s/mm2 is recommended for the estimation and interpretation of FD measures 10. Second, the sample size is relatively small, which needs to be taken into consideration while interpreting our results. Finally, we did not investigate the impact of the presence of white matter hyperintensities, which might impact our results. Our current and future work will be to examine the longitudinal trajectory of whole-brain as well as tract of interest specific alterations in FBA measures in MCI and AD.Conclusion
This study highlights the potential of FBA to detect fibre-specific changes in the WM microstructure related to AD. Future longitudinal FBA studies including early onset cohort with less clinical heterogeneity are needed to elucidate where the disease starts and how it progresses in the brain. Ongoing methodical improvements in FBA will improve the value of this tool, particularly by evaluating its performance in the presence of pathologies including large focal lesions, and white matter hyperintensities.Acknowledgements
This project was supported by P30 AG072972 grant. The data included in this study were provided by UC Davis Alzheimer's Disease Research Center.
References
1. Clerx L, Visser PJ, Verhey F, Aalten P. New MRI markers for Alzheimer's disease: a meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. Journal of Alzheimer's Disease 2012;29:405-429.
2. Jack Jr CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. The lancet neurology 2013;12:207-216.
3. Raffelt DA, Tournier J-D, Smith RE, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage 2017;144:58-73.
4. Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage 2007;35:1459-1472.
5. Tournier J-D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 2019;202:116137.
6. Dhollander T, Mito R, Raffelt D, Connelly A. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. Proc Intl Soc Mag Reson Med; 2019.
7. Raffelt DA, Smith RE, Ridgway GR, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage 2015;117:40-55.
8. Xiao D, Wang K, Theriault L, Charbel E, Initiative AsDN. White matter integrity and key structures affected in Alzheimer's disease characterized by diffusion tensor imaging. European Journal of Neuroscience 2022;56:5319-5331.
9. Mito R, Raffelt D, Dhollander T, et al. Fibre-specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain 2018;141:888-902.
10. Raffelt D, Tournier JD, Rose S, et al. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. NeuroImage 2012;59:3976-3994.
Figures