3516
APOE ε4 and Hypertension: Cardiovascular Risk Accelerate the Brain Structure Atrophy and Small Vessel Disease Pathologies in MCI and Dementia1Department of Biological Sciences, Indian Institute of Science Education and Research, Berhampur, Berhampur, India
Synopsis
Keywords: Dementia, Aging, White Matter Hyperintensity, APOE E4, Cognition, Hypertension
Transformation of brain heath with aging comprehend a series of brain structure, vascular and functional changes over time. The key to understanding the brain health in normal and pathological aging is to identify the series of early, intermediate and late events that encodes for brain health. Here, we have investigated the temporal and spatial orders of brain volumetry, small vessel pathology (white matter hyperintensity) and its kinetics with normal aging and aging with cardiovascular risks and gene defects such as APOE ε4, across subjects classified as cognitively Normal (CN), Mild Cognitive Impairment (MCI) and Dementia (DM).Summary
Cardiovascular risks such as hypertension and APOE ε4 allele have a profound effect on brain volumetric changes. Hypertensive subjects show increased white matter hyperintensity load with aging compared to normotensive subjects. APOE ε4 allele alters the kinetics of gray matter, hippocampus, and CSF volume.Introduction
The human brain undergoes inevitable structure and functional changes with aging. The subtle brain structure changes during the course of normal aging overlap to a large extent with the brain volumetric and surface changes associated with cognitive impairment and dementia. Aging-associated brain health changes may get altered manifold with comorbid cardiovascular risks such as hypertension1-3. Secondly, gene defects also play a major role in the pathogenesis of degenerative disorders of the nervous system. But there is a need to delineate the difference between normal aging and pathological conditions accompanying aging like Alzheimer's disease. Apolipoprotein ε4 (APOE ε4) is a risk condition that perturbs the intracellular lipid state and results in increased unsaturation of fatty acids and accumulation of intracellular lipid droplets4. Therefore, this dysregulation of lipids has emerged as a key feature of several neurodegenerative diseases in humans5-6. Here, we have performed a comprehensive neuroimaging analysis to investigate the effects of hypertension and APOE ε4 allele on brain structure morphometry and brain functional connectivity, with cognition in cognitively normal, mild cognitive impairment, and dementia subjects of Alzheimer’s Disease Neuroimaging Initiative (ADNI) study.Methods
Brain segmentation was performed on T1, T2, and T2-FLAIR MR images to determine the volume of brain structures and White Matter Hyperintensity (WMH) load using Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort with a total of 5102 longitudinal-MRI scans. Changes in the different brain structure volumes with age were investigated across Cognitively normal (CN, scans = 2038, subjects = 745), Cognitively Impaired (CI, scans= 2515, subjects = 862), and Dementia(DM scans =549, subjects = 281) groups. Subjects enrolled in the study were evaluated for cognitive status using the global Clinical Dementia Rating (CDR) score. Based on an individual’s APOE allele status, subjects groups were further divided into two genotype subgroups – ε4 carriers (at least one APOE ε4 allele) and ε4 non-carriers (no APOE ε4 allele). Multivariate analyses were performed to understand the association of change in structural volumetry and cerebrovascular risk factors with APOE status in different cognitive groups. Multivariate linear regression with structural volumes as the dependent variable and age, hypertension, and APOE4 status as independent variables were performed. Individual effects as well as interaction effects of hypertension, APOE ε4 status, and age on brain structural volumetry were further explored using regression analysis methods in R and Python.Results and Discussion
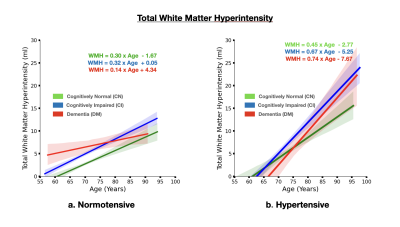
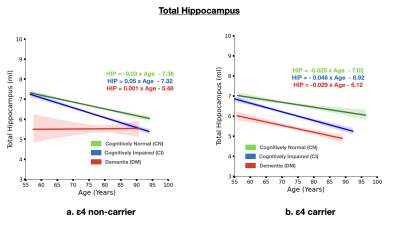
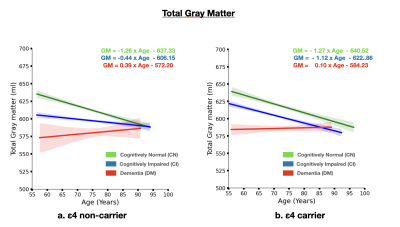
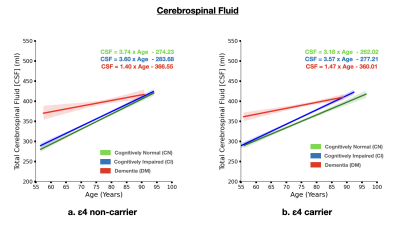
The brain MRI changes in DM subjects compared to MCI and CN groups at different age groups were also identified as early, intermediate and late brain MRI changes.Total White Matter Hyperintensity (WMH) across hypertensive and normotensive subjects in the cognitively normal, cognitively impaired and Alzheimer’s disease group was found to be significantly elevated in the hypertensive subjects (p<0.05) (Fig. 1) The rate of increase of WMH load with age is significantly higher in MCI and DM subjects compared to CN.The brain MRI changes in DM subjects compared to MCI and CN groups at different age groups were also identified as early, intermediate and late brain MRI changes Subjects with ε4 carriers showed a significantly faster loss in the hippocampus volume with age compared ε4 non-carriers in MCI and DM subjects (p<0.05) (Fig. 3). In addition to this ε4 carriers showed similar kinetics for gray matter and cerebrospinal fluid volume. Clearly, subjects with ε4 carrier showed significant loss of gray matter in MCI subjects (p<0.05) (Fig. 4). In addition to this cognitively normal subjects showed significant increase in cerebrospinal fluid or CSF volume with APOE ε4 allele. (p<0.05) (Fig. 5)Conclusion
Concurrent occurrence of hypertension and ε4 carriers has a superficial effect on WMH increase and ventricular hypertrophy with aging than isolated events of hypertension or APOE ε4 allele. This further intrigues to study the effect of other cardiovascular risk factors such as diabetes and smoking on brain structure atrophy and cerebral small vessel disease pathologies in different cognitive groups.Acknowledgements
MRI, cognitive data and APOE status was obtained from the Alzheimer’s Disease Neuroimaging Initiative database(ADNI) funded by National Institutes of Health (Grant U19 AG024904)References
1. Kern KC, Wright CB, Bergfield KL, Fitzhugh MC, Chen K, Moeller JR, et al. Blood pressure control in aging predicts cerebral atrophy related to small-vessel white matter lesions. Front Aging Neurosci. 2017;9:132. doi: 10.3389/fnagi.2017.00132.
2. Muscari A, Faccioli L, Ghinelli M, Napoli C, Pirazzoli E, Puddu GM, et al. Hypertension and other determinants of white matter lesions in stroke patients. J Clin Hypertens (Greenwich) 2016;18:907–12. doi: 10.1111/jch.12788.
3. Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–98. doi: 10.1038/nrcardio.2010.161.
4. Mahley, R. W. & Rall, S. C. Jr. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 (2000).
5. Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015 Jul 16;11:1723-37. doi: 10.2147/NDT.S84266. PMID: 26213471; PMCID: PMC4509527.
6. Lahoz, C. et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis 154, 529–537 (2001).
Figures