3511
Relating left hippocampal microstructure and delayed memory in cognitively intact adults at genetic-risk for developing Alzheimer's disease1School of Biological and Health Systems Engineering, Arizona State University, Tempe, AZ, United States, 2Psychiatry and Psychology, Mayo Clinic, Scottsdale, AZ, United States, 3Radiology, Mayo Clinic, Scottsdale, AZ, United States, 4Neurology, Mayo Clinic, Scottsdale, AZ, United States, 5Radiology, Vanderbilt University, Nashville, TN, United States
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease, ApoE4; genetic-risk
We have recently shown that the functional connectivity of the left hippocampus is significantly related to memory trajectory in cognitively intact ApoE4 carriers, independent of hippocampal volume. The purpose of this preliminary study was to determine if dendritic orientation dispersion of the left hippocampus explains variance in delayed verbal memory in this cohort. Results indicated that dendritic complexity of the left hippocampus was related to delayed memory in ApoE4 carriers. These findings suggest that ApoE4 carriers may experience subtle microstructural declines in the left hippocampus at the cognitively-intact stage.Introduction
The ApoE4 allele is the greatest genetic risk factor for developing Alzheimer’s disease (AD) and is thought to exacerbate synaptic loss1,2. The disconnection and asymmetry hypotheses of AD pathogenesis posit that the hippocampus becomes functionally disconnected from neural networks due to synaptic loss and that this pathology begins within left hemispheric structures (such as the hippocampus)3. In support of these hypotheses, we have recently shown that the functional connectivity of the left hippocampus was significantly related to memory trajectory in cognitively intact ApoE4 carriers, independent of hippocampal volume. Given that ApoE4 carriers may experience exacerbated synaptic loss, it is plausible that functional disconnection of the hippocampus may be due to a loss of synaptic connections. The orientation dispersion index (ODI) is a measure of dendritic coherence4 (i.e., low dispersion suggests low dendritic complexity), and a recent report suggests ODI is positively correlated with a PET biomarker of synaptic density5. The purpose of this study was to determine if ODI of the left hippocampus explains variance in delayed verbal memory in cognitively intact ApoE4 carriers. We hypothesized that carriers would have lower left hippocampal ODI than noncarriers, and that left hippocampal ODI would demonstrate a positive relationship with delayed recall scores in the carrier group.Methods
Participants completed the delayed recall portion of the Rey Auditory and Verbal Learning Test (ref) and underwent multi-shell diffusion imaging on a 3T MRI scanner at the Mayo Clinic, Scottsdale, AZ. Diffusion acquisition parameters included: b-value = 1, and 2 ms/µm2 with 38 and 47 diffusion encoding directions, respectively, and nine interleaved B0s. Raw diffusion data were preprocessed to reduce noise and the neurite orientation and dispersion and density imaging model6 was applied to quantify ODI in each voxel. Bilateral hippocampal regions were identified on the standard MNI Atlas and were coregistered to participant native diffusion space using FSL (FMRIB, Oxford). Participant memory scores were included as a predictor variable in separate linear regression models to predict the ODI of left and right hippocampus; age was added as a covariate to account for individual differences in tissue microstructure due to aging. Recent neuroimaging studies suggest that hippocampal subfields (e.g., the subiculum, CA1, etc.) may be the first neuroanatomical regions to demonstrate AD-related pathology associated with memory decline7; secondary analyses examined whether ODI of specific subfields were related to memory. Hippocampal subfield regions of interest were first aligned to an older adult brain atlas and then aligned to participant native diffusion space. Participant age and memory scores were included as predictor variables in regression models designed to predict the ODI of each subfield region of interest. An FDR-correction was applied to account for multiple statistical tests.Results
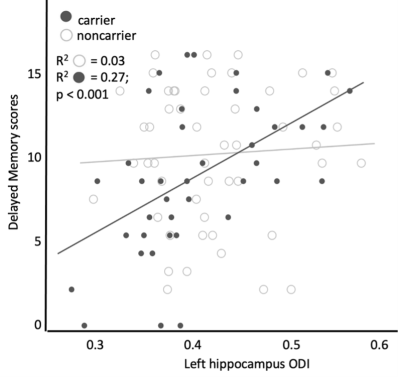
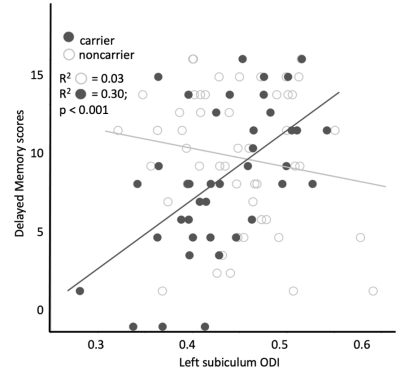
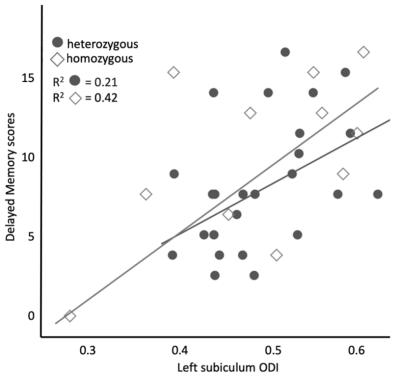
The carrier group demonstrated a significant relationship between delayed verbal memory scores and left hippocampal ODI (β=0.007; p<0.001, Fig. 1). When age was included as a covariate, the ODI of the subiculum demonstrated a significant positive relationship with delayed verbal memory scores in the carrier group (FDR-corrected p=0.002, Fig. 2). There were no significant differences observed between carrier and noncarrier groups in delayed verbal memory scores nor the left and right hippocampal ODI. Because the ApoE4 allele demonstrates a ‘dose effect’ in the age of onset (i.e., homozygous carriers develop AD earlier in life than heterozygous carriers)8, follow-up analyses evaluated if a ‘dose effect’ of zygosity existed between carriers, heterozygous, and homozygous carriers. Homozygous carriers demonstrated a trend of lower left hippocampal ODI than noncarriers (p=0.09) and no difference was observed between heterozygous carriers and other groups. Moreover, homozygous carriers demonstrated a stronger relationship between left subiculum ODI and delayed verbal memory scores compared to heterozygous carriers, although this difference did not reach the threshold of statistical significant (Fig. 3).Discussion and Conclusions
Our results suggest that delayed verbal memory performance may be linked to dendritic microstructure of the left subiculum and that ApoE4 carriers may experience subtle microstructural declines in this neural region at the cognitively intact stage. While ODI has been linked to a PET biomarker of synaptic density, it lacks histological validation against ground-truth measures of synapse structure. Future work will histologically validate ODI as a biomarker of synapse density in rodent models.Acknowledgements
References
1. Lane-Donovan, C. & Herz, J. ApoE, ApoE Receptors, and the Synapse in Alzheimer’s Disease. Trends Endocrinol Metab28, 273–284 (2017).
2. Hesse, R. et al. Comparative profiling of the synaptic proteome from Alzheimer’s disease patients with focus on the APOE genotype. Acta Neuropathol Commun 7, 214 (2019).
3. Mohtasib, R. et al. MRI biomarkers for Alzheimer’s disease: the impact of functional connectivity in the default mode network and structural connectivity between lobes on diagnostic accuracy. Heliyon 8, e08901 (2022).
4. Colgan, N. et al. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer’s disease. Neuroimage 125, 739–744 (2016).
5. Mak, E. et al. In vivo coupling of dendritic complexity with presynaptic density in primary tauopathies. Neurobiol Aging101, 187–198 (2021).
6. Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016 (2012).
7. Abraham, M. et al. Episodic Memory and Hippocampal Volume Predict 5-Year Mild Cognitive Impairment Conversion in Healthy Apolipoprotein ε4 Carriers. Journal of the International Neuropsychological Society 26, 733–738 (2020).
8. Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–56.
Figures