3510
Detecting micro- and macro-structural deviations in individuals with cognitive impairment1VUMC, Nashville, TN, United States, 2Sherbrooke University, Sherbrooke, QC, Canada, 3Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 4Vanderbilt University, Nashville, TN, United States, 5National Institute on Aging, Baltimore, MD, United States, 6Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease
Medical imaging is a promising tool in detecting altered brain tissue states related to cognitive impairment and dementia, however, clinical heterogeneity challenges interpretation of these changes. Recent work in normative modeling has paved the way for not only group-based comparisons of control/cohorts, but detection of deviations in individual subjects. Here, we apply this framework to detect anomalies in individuals with cognitive impairment by assessing the classification power of microstructural and macrostructural features of different tissue types, and also attributing anomalies to specific features of brain tissue.Introduction
Neuroimaging features have shown promising ability to detect altered tissue states related to cognitive impairment and dementia. For example, structural images provide measures of cortical macrostructure and deep gray matter (GM) volumetry that show differences between cognitively normal (CN) populations and those with cognitive impairment, are strongly related to neurocognitive function, and may be predictors of future impairment [1-5]. On the other hand, diffusion MRI provides measures of white matter (WM) microstructure that have similarly shown group differences, associations with cognition, and alterations in preclinical stages [6-9].However, the large dimensionality and heterogeneity of these neuroimaging features can challenge statistical group-wise comparisons and biological interpretation of results. A further unmet challenge is to identify altered tissue states at the individual level. Towards this end, a normative modeling [10] technique called “Detect” [11] was developed to capture patterns of features from highly dimensional datasets to detect anomalies in individuals that differ from a healthy population. We aim to extend this framework to detect anomalies in individuals with cognitive impairment. While this technique was originally developed to detect deviations in microstructure along white matter pathways, we extend the neuroimaging features used as input, while also including features unexplored in the cognitive impairment literature, including microstructure and macrostructural features of white matter, gray matter, and deep gray matter. We use the framework to assess the discriminative power of different feature sets, to identify anomaly scores for individual subjects, and to identify features of the brain that are most frequently atypical in cognitive impairment.
Methods
DataThis study uses a subset of data from the Baltimore Longitudinal Study of Aging (BLSA) dataset. We have chosen only a cross-sectional sample of subjects, including 52 diagnosed with mild-cognitive impairment (MCI) or Alzheimer’s Disease (AD) at the time of scanning, and 208 cognitively normal age/sex matched controls. Diffusion MRI data was acquired on a 3T Philips Achieva scanner (32 gradient directions, b-value=700s/mm2, TR/TE=7454/75ms, reconstructed voxel size=0.81×0.81×2.2mm).
Features
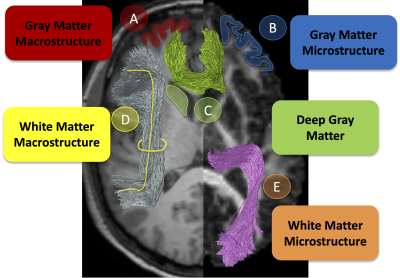
This study used five different types of features, visualized in Figure 1. First, GM macrostructural features were derived from FreeSurfer [12] and mapped to the Desikan Killey [13] parcellation resulting in 8 features (cortical thickness, volume, curvature, etc.) for each of 84 regions. Second, GM microstructural features from diffusion tensor imaging (DTI) were derived for the same parcellation resulting in 4 features (fractional anisotropy, mean, axial, radial diffusivities) for 84 regions. Third, deep GM volumes derived from FreeSurfer were utilized and described by 5 features (volume and DTI measures) for 16 regions. Fourth, white matter microstructure features were derived from DTI and mapped to 39 TractSeg [14] derived pathways. Finally, 21 white matter macrostructure measures (including volumes, areas, lengths, see [15] for details) were derived for the same 39 pathways.
Detection
The detection framework described in [11] was used in this study. Briefly, Detect uses an autoencoder to create a low dimensional feature space to learn normative patterns of features across a healthy population. By projecting an individual subject through this autoencoder, an anomaly score can be calculated based on reconstruction error from the normative population. From this, we calculated the average AUC computed on[MC1] the same number of subsets of CN subjects, showed the distribution of anomaly scores of individual subjects, and rank the number of anomalies identified for each feature. [MC1]Try to say "same number of" or something like this. It has to be clear that we computed AUCs correctly. Matching subset is nice and can be kept but maybe people only think of it as in age/sex.
Results
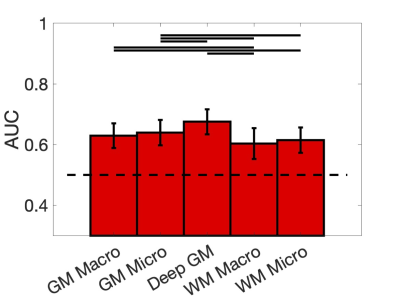
Figure 2 shows the discriminating power of the Detect network as the average AUC over 100 bootstrap iterations for each feature set. While discriminative power is moderate, all are above chance, with highest AUC for deep GM and GM microstructure features.Figure 3 show the anomaly scores for individual MCI/AD subjects against the CN group for each set of features. In general, there is a higher reconstruction error for the MCI/AD subjects, although significant overlap exists.
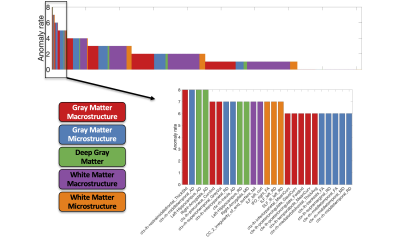
Figure 4 shows the anomaly rates of individual features from the AD/MCI dataset. Notably, the features are highly heterogenous, with a maximum occurrence of 8 (out of 52 subjects), although GM and deep GM features tend to occur more frequently.
Discussion
We have applied the Detect framework to a cohort with cognitive impairment. While this is a challenging task due to the biological heterogeneity of the disease between individuals, we find that we can detect anomalies that occur in different features of the brain and for different individuals. On a group level, we find a low/moderate discriminative ability to detect MCI/AD with tissue-specific feature sets, and that deep GM and GM macrostructure features may have higher sensitivity/specificity to pathology. On an individual level, MCI/AD subjects tend to have higher overall anomaly scores, and individual anomalous features identified using the Detect framework are highly heterogenous across the cognitively impaired population.Future work should include (1) utilizing BLSA dataset to detect preclinical stages of dementia on scans prior to diagnosis, (2) relating anomaly scores to cognition, (3) including additional features including superficial white matter [16], and (4) investigating generalizability of the Detect framework across datasets.
Acknowledgements
This work was supported by the National Science Foundation Career Award #1452485, the National Institutes of Health under award numbers R01EB017230, K01EB032898, and in part by ViSE/VICTR VR3029 and the National Center for Research Resources, Grant UL1 RR024975–01. MC was supported by the Radboud Excellence Initiative Fellowship.References
1. Tuokkola, T., et al., Association between Deep Gray Matter Changes and Neurocognitive Function in Mild Cognitive Impairment and Alzheimer’s Disease: A Tensor-Based Morphometric MRI Study.Dementia and Geriatric Cognitive Disorders, 2019. 48(1-2): p. 68-78.
2. Pini, L., et al., Brain atrophy in Alzheimer's Disease and aging. Ageing Res Rev, 2016. 30: p. 25-48.
3. Cho, H., et al., Shape changes of the basal ganglia and thalamus in Alzheimer's disease: a three-year longitudinal study. J Alzheimers Dis, 2014. 40(2): p. 285-95.
4. Armstrong, N.M., et al., Associations between cognitive and brain volume changes in cognitively normal older adults. Neuroimage, 2020. 223: p. 117289.
5. Busovaca, E., et al., Is the Alzheimer's disease cortical thickness signature a biological marker for memory? Brain Imaging Behav, 2016. 10(2): p. 517-23.
6. Shafer, A.T., et al., Accelerated decline in white matter microstructure in subsequently impaired older adults and its relationship with cognitive decline. Brain Commun, 2022. 4(2): p. fcac051.
7. Williams, O.A., et al., Vascular burden and APOE epsilon4 are associated with white matter microstructural decline in cognitively normal older adults. Neuroimage, 2019. 188: p. 572-583.
8. Reginold, W., et al., Altered Superficial White Matter on Tractography MRI in Alzheimer's Disease.Dement Geriatr Cogn Dis Extra, 2016. 6(2): p. 233-41.
9. Fieremans, E., et al., Novel white matter tract integrity metrics sensitive to Alzheimer disease progression. AJNR Am J Neuroradiol, 2013. 34(11): p. 2105-12.
10. Marquand, A.F., et al., Understanding Heterogeneity in Clinical Cohorts Using Normative Models: Beyond Case-Control Studies. Biol Psychiatry, 2016. 80(7): p. 552-61.
11. Chamberland, M., et al., Detecting microstructural deviations in individuals with deep diffusion MRI tractometry. Nat Comput Sci, 2021. 1: p. 598-606.
12. Fischl, B., FreeSurfer. Neuroimage, 2012. 62(2): p. 774-81.
13. Desikan, R.S., et al., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 2006. 31(3): p. 968-80.
14. Wasserthal, J., P. Neher, and K.H. Maier-Hein, TractSeg - Fast and accurate white matter tract segmentation. Neuroimage, 2018. 183: p. 239-253.
15. Yeh, F.C., Shape analysis of the human association pathways. Neuroimage, 2020. 223: p. 117329.
16. Shastin, D., et al., Short Association Fibre Tractography. bioRxiv, 2021: p. 2021.05.07.443084.
Figures