3502
Dynamic associations between tonic alertness and brain activity during sleep inertia1Department of Radiology, Tri-Service General Hospital, Taipei, Taiwan, 2Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei, Taiwan, 3National Defense Medical Center, Taipei, Taiwan, 4McConnell Brain Imaging Center, Montreal Neurological Institute, McGill University, Montréal, QC, Canada, 5Department of Counseling and Industrial/Organizational Psychology, Ming Chuan University, Taoyuan, Taiwan, 6Graduate Institute of Mind, Brain and Consciousness, Taipei Medical University, Taipei, Taiwan, 7Brain and Consciousness Research Center, Taipei Medical University-Shuang Ho Hospital, New Taipei, Taiwan
Synopsis
Keywords: Brain Connectivity, fMRI (task based)
Sleep inertia (SI) occurs during the transition from sleep to waking, accompanied by temporary hypoalertness and decreased tonic alertness around half an hour. We used the psychomotor vigilance task (PVT) to detect the dynamic alteration of tonic alertness during SI by monitoring the Cingulo-opercular network activity through simultaneous EEG-fMRI scanning. The alteration was greater when comparing the Pre PVT session to early wakefulness than to the late wakefulness, and was easily detectable under the task demands. A dynamic of tonic alertness could be observed during SI under the demands of the task.Introduction
Sleep inertia (SI) occurs during the transition from sleep to waking, accompanied by temporary hypoalertness and decreased tonic alertness around half an hour (Tassi and Muzet 2000). Resting-state fMRI studies disclosed the slow process of brain reorganization after sleep (Tsai et al. 2014); however, a missing link remains to be uncovered between the declined behavioral task performances and the specific brain mechanisms during SI. Therefore, we used psychomotor vigilance task (PVT) to measure the dynamic changes of tonic alertness and the associated brain activity, which target on Cingulo-opercular network (CON) through fMRI scanning.Methods
Adjusted to the regular sleep time, 26 participants were divided into two groups according to their awaking state: inertia (N=15) and control (N=11). Four PVT tasks were performed (once before sleeping as Pre, and three times after sleeping as A1 to A3) with simultaneous EEG-fMRI recordings.Results
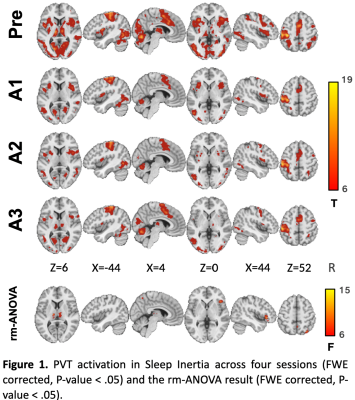
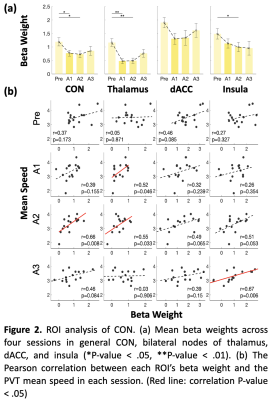
A significant time effect of PVT activity was mainly found in the bilateral thalamus inside the CON (Figure 1). SI group showed a main effect of time on the CON network, with its activation significantly higher during the Pre PVT session compared with A1 and A2, but not with A3 during late wakefulness (Figure 2a). Bilateral thalamus activation declined in A1 and A2 but increased in A3. There is no significant difference in activation levels of PVT-demanded stimuli in the Control group.Furthermore, CON activation correlated positively with PVT speed in late wakefulness (A2, marginally at A3), while during early wakefulness (A1 to A2), thalamus activation correlated with PVT alertness measurement (Figure 2b). This correlation-based dynamic pattern has not appeared in the Control group.
Discussion
The tonic alertness of CON was achieved by suppressing distracting information originating from both the external and internal environments (Sadaghiani and D’Esposito 2015). In late post-sleep wakefulness (A2, marginally in A3), the higher CON activation was associated with faster PVT speed. It may reflect the increased flexibility of CON during the time when subjects were responding to stimuli with effort, thereby demonstrating the positive impact of taking a nighttime nap from a neural perspective. Interestingly, the absent correlation in early wakefulness, indicating the uncoupling of CON activity with behavioral performance, could be one of the neural signatures impacted by SI, which is consistent with previous EEG studies (Sadaghiani and Kleinschmidt 2016; Sepúlveda, Tapia, and Monsalves 2019). Whereas the broken correlations in the Control group may be due to the accumulation of sleepiness because of the long duration of staying awake at midnight.Conclusion
Under PVT during SI, we observed task-specific aspects of neurophysiological dynamics and dynamic changes in CON activity. The alteration presented stronger under the high demand PVT response in SI. A dynamic of tonic alertness could be observed during SI under the demands of the task.Acknowledgements
This research was funded by Taiwan National Science and Technology Council (NSTC 111-2314-B-038-063, NSTC 111-2321-B-A49-003, NSTC 111-2321-B-A49-006).References
Sadaghiani, Sepideh, and Mark D’Esposito. 2015. “Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness.” Cerebral Cortex 25 (9): 2763–73. https://doi.org/10.1093/cercor/bhu072.
Sadaghiani, Sepideh, and Andreas Kleinschmidt. 2016. “Brain Networks and α-Oscillations: Structural and Functional Foundations of Cognitive Control.” Trends in Cognitive Sciences 20 (11): 805–17. https://doi.org/10.1016/j.tics.2016.09.004.
Sepúlveda, P. O., L. F. Tapia, and S. Monsalves. 2019. “Neural Inertia and Differences between Loss of and Recovery from Consciousness during Total Intravenous Anaesthesia: A Narrative Review.” Anaesthesia 74 (6): 801–9. https://doi.org/10.1111/anae.14609.
Tassi, Patricia, and Alain Muzet. 2000. “Sleep Inertia.” Sleep Medicine Reviews 4 (4): 341–53. https://doi.org/10.1053/smrv.2000.0098.
Tsai, Pei-Jung, Sharon Chia-Ju Chen, Chun-Yao Hsu, Changwei W. Wu, Yu-Chin Wu, Ching-Sui Hung, Albert C. Yang, Po-Yu Liu, Bharat Biswal, and Ching-Po Lin. 2014. “Local Awakening: Regional Reorganizations of Brain Oscillations after Sleep.” NeuroImage 102 (November): 894–903. https://doi.org/10.1016/j.neuroimage.2014.07.032.
Figures