3500
Effects of Abnormal Brain Network Connectivity after Sleep Deprivation Based on Intra-Individual Variance1Department of Radiology, Xijing Hospital, Fourth Military Medical University, Xi'an, China
Synopsis
Keywords: Brain Connectivity, fMRI (task based)
Sleep deprivation (SD) can cause task performance and negative correlation strength between default mode (DM) and task related network decreasing, but the detailed mechanism is still unclear. Intra-individual variance (IIV) is thought to associated with the negative correlation strength between DM and task active network. Thus, in this study, IIV was used to evaluate the relationship between the changing of this negative correlation strength and the task performance after SD. Results showed that SD may increase the healthy subjects’ IIV through reducing the competition relationship between DM and task active network, which resulted in the temporal impairments of cognitive function.ABSTRACT
INTRODUCTIONSleep deprivation (SD) is becoming increasingly common in our modern “24/7” society [1], which can cause various consequences, including sleepiness, impaired cognitive, and even industrial, transportation and medical accidents [2]. Currently, many studies have been reported that the performance of cognitive function is associated with negative correlation between default mode (DM) network and task related brain regions [3-4]. Although SD can cause both task performance and this negative correlation strength decreasing [5], the detailed mechanism between them is still unclear. Intra-individual variance (IIV), which is calculated as the changing of task related indices between different situations, is thought to reflect the efficiency with which attentional resources are allocated in different cognitive control circumstances [6]. It has been pointed out that IIV is associated with the negative correlation strength between task active network and DM network [4]. Thus, in this study, we used IIV derived from psychomotor vigilance task (PVT) to evaluate the relationship between the changing of cognitive function and the negative correlation strength between task active network and DM network.
METHOD
Seventy right-handed healthy college students were recruited in our study. After selected by exclusion criteria and data pre-processing, data from sixty-seven subjects were finally used. All subjects underwent an fMRI scan with PVT after normal sleep (NS) and after SD, respectively, with a scan interval at least one week. Two analysis modes, named network-level and region-level independent component analysis (NL-ICA and RL-ICA, respectively) were used. In NL-ICA, 25 independent components (ICs) were extracted across all subjects and all sessions clustered into 8 resting-state networks (RSNs) based on Yeo atlas [7]. The task active network was constructed using general linear model (GLM) method based on event-related task design, i.e., PVT task. In RL-ICA, 20 independent components (ICs) were extracted with DM components and task active network from NL-ICA as mask. Two-tailed paired T test was used to evaluate the changing between NS and SD. Pearson correlation of those pairs of functional connectivity (FC) that significant changing after SD between RSNs and task active network with PVT response time (RT) and coefficient of variance (CV) were calculated. A p-value less than 0.05 was considered as statistical significance.
RESULTS
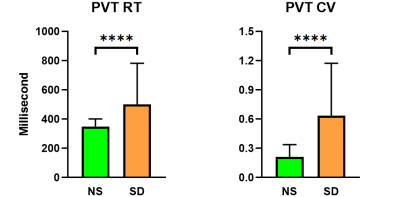
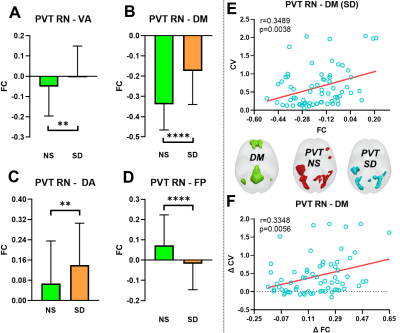
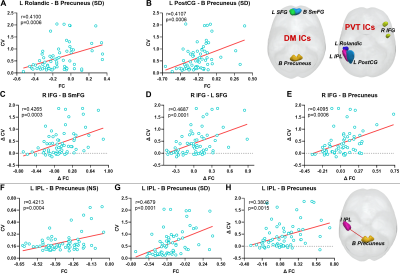
Compared with NS, the PVT RT value and CV was significantly increased after one night SD (all p < 0.0001, Fig 2). For NL-ICA, FCs between PVT-related network and DM, attention and frontal-parietal networks were significant changing after SD (all p < 0.005, Fig 3A-3D), but only the negative FC strength between PVT-related network and DM network was significantly positively correlated with PVT CV after SD (r = 0.3489, p = 0.0038, Fig 3E). Moreover, the changing between these two metrics was also significantly positively correlated (r = 0.3348, p =0.0056, Fig 3F). Furthermore, for RL-ICA, FC of left inferior parietal lobe (L IPL) - bilateral precuneus was significantly positively correlated with PVT CV after NS, FC of L postcentral gyrus (L PostCG)- bilateral precuneus, L Rolandic - bilateral precuneus and L IPL - bilateral precuneus were significantly correlated with PVT CV after SD, and the FC changing of right inferior frontal gyrus (R IFG) - bilateral superior median frontal gyrus, R IFG - L superior frontal gyrus, R IFG - bilateral precuneus and L IPL - bilateral precuneus were significantly correlated with PVT CV changing (all p < 0.002, Fig. 4).
DISCUSSION
After NS, each brain network worked well. Different with Kelly et al [4] study, the negative FC strength between DM and PVT-related network didn’t correlate with PVT CV for NL-ICA. But for RL-ICA, we found FC between L IPL and bilateral precuneus was correlated with PVT CV. This may be due the ceiling effect. Based on the fact that PVT task is relatively an easy task, the coarse brain region segment (i.e., NL-ICA) may not catch this important relationship. After SD, normal brain cognitive functions were seriously impaired, which could be reflected by the significant FC changing between PVT-related network and DM, attention and Frontal-Parietal networks. Our results showed that the DM-PVT FC strength was positively correlated with PVT CV, as well as the changing between them (both NL-ICA and RL-ICA). These results may suggest that the reduced DM-PVT FC strength will cause the functional impairment of these two networks and the interaction between them, which may increase the subjects’ IIV and finally cause the cognitive function impairment. Interestingly, the FC of L IPL and bilateral precuneus was positively correlated with PVT CV after both NS and SD, as well as the changing between them, which may provide great information for the mechanism among SD, IIV and temporal impairments of cognitive function.
CONCLUSIONS
In summary, SD may increase the healthy subjects’ IIV through reducing the competition relationship between task active network (in this study, PVT network) and DMN, and thus, resulted in the temporal impairments of cognitive function.
Acknowledgements
We thank all those who have helped us during the designing and writing of this abstract.References
[1] Geiker NRW, Astrup A, Hjorth MF, Sjödin A, Pijls L, Markus CR (2018). Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes. Rev 19:81–97. https://doi.org/10.1111/obr.12603.
[2] Atrooz F, Salim S (2020). Sleep deprivation, oxidative stress and inflammation. Adv Protein Chem Struct Biol 119:309-336. https://doi.org/10.1016/bs.apcsb.2019.03.001.
[3] Weissman, D.H., Roberts, K.C., Visscher, K.M. and Woldorff, M.G. The neural bases of momentary lapses in attention. Nat. Neurosci., 2006; 9: 971-978. doi: 10.1038/nn1727.
[4] Kelly, A.M., Uddin, L.Q., Biswal, B.B., Castellanos, F.X. and Milhamm M.P. Competition between functional brain networks mediates behavioral variability. NeuroImage, 2008, 39: 527-537. doi: 10.1016/j.neuroimage.2007.08.008.
[5] Zhu Y, Xi Y, Sun J et al. Neural correlates of dynamic changes in working memory performance during one night of sleep deprivation. Hum Brain Mapp. 2019, 40(11): 3265-3278. doi: 10.1002/hbm.24596.
[6] Jensen, A.R. The importance of intraindividual variation in reaction time. J. Pers. Individ. Differ., 1992, 13: 869-881.
[7] Yeo BT, Krienen FM, Sepulcre J et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011 Sep;106(3):1125-65. doi: 10.1152/jn.00338.2011.
Figures