3498
Mood repair in long-term meditators is predicted by rsfMRI functional connectivity of the insula and correlates with FA of the uncinate fasciculus1Institute of Information and Communication Technologies, Electronics and Applied Mathematics, Université Catholique de Louvain, Louvain La Neuve, Belgium, 2Institute Of NeuroScience, Université Catholique de Louvain, Bruxelles, Belgium
Synopsis
Keywords: Brain Connectivity, Brain
We used rsfMRI and diffusion imaging to study functional and structural brain changes underlying the psychological trait mood repair in long-term meditators. Results of connectome-based predictive modeling showed that measured “mood repair” scores in meditators could be successfully predicted from rsfMRI data during meditation. The highest degree node in the underlying network was in the anterior ventral insula. Diffusion imaging data further revealed a role of the uncinate fasciculus in the mood repair trait in meditators. The uncinate fasciculus is part of the limbic network, connecting anterior temporal lobe with the inferior prefrontal cortex.Introduction
There is now ample evidence that meditation may strongly affect the brain functional and structural connectivity1. However, most of these studies have been conducted in participants with only limited meditation experience and lack psychological profiling of the meditators. The aim of the present study was to use resting state fMRI (rsfMRI) and diffusion imaging (DI) in combination with psychological profiling in a large group of highly experienced meditators in order to assess long-term changes in brain structure and function and how these relate to psychological variables.Methods
Participants.Fifty meditators (44.0±5.6$$$\,$$$y;$$$\,$$$24F) and 37$$$\,$$$age-$$$\,$$$and sex-matched non-meditator controls (44.2±7.5$$$\,$$$y;$$$\,$$$21F). Mean number of years of meditation practice: 17.2±6.2$$$\,$$$y (range$$$\,$$$6-29$$$\,$$$y). Care was taken to match controls and meditators for life style factors that could affect results.
Psychological profiling.
Participants filled in a number of personality questionnaires to assess dispositional traits including interoceptive awareness, emotional coping, anxiety, depression, and impulsiveness. “Mood Repair” was assessed using the Trait-Meta Mood Scale$$$\,$$$(TMMS)2.
MRI scanning.
Participants were scanned on a 3T$$$\,$$$Philips$$$\,$$$Achieva scanner. The MRI session included one anatomical sequence$$$\,$$$(T1-weighted), one eyes-closed rsfMRI scan, one Diffusion Imaging (DI) sequence, and one fMRI scan with participants in a meditative state. The T1-weighted anatomical image was obtained with a gradient-echo sequence with an inversion prepulse acquired in the sagittal plane with TR=9.1ms,$$$\,$$$TE=4.6ms,$$$\,$$$flip angle=8°,$$$\,$$$150 slices, slice thickness=1mm, in-plane resolution reconstructed in 0.75x0.75$$$\,$$$mm2. DI images were acquired using a spin$$$\,$$$echo planar sequence: TE=83ms,$$$\,$$$TR=6422ms,$$$\,$$$70$$$\,$$$slices, slice thickness=2mm, in-plane resolution=2x2mm2,$$$\,$$$55$$$\,$$$directions. A reference b0 image and one b=800$$$\,$$$s/mm2 image were acquired. Resting-state and meditation fMRI images were acquired using repeated single-shot echo-planar imaging: TE=30ms,$$$\,$$$FA=90°, in plane resolution=3.44x3.44mm2,$$$\,$$$35$$$\,$$$slices, slice thickness=3.44mm, TR=2000ms and number of TR=200$$$\,$$$(acquisition length=6min40s).
MRI Preprocessing.
Preprocessing of the fMRI data included linear trend removal to exclude scanner-related signal drift, temporal high-pass filter to remove frequencies lower than 0.005$$$\,$$$Hz and correction for head movements. Data was corrected for slice-timing differences, co-registered to the T1w-reference and normalized in the MNI space. Additional preprocessing steps were added to remove non-neural artifacts from the BOLD signals. Regression analyses were performed to remove artifacts due to residual motion (the six movement regressors were obtained via rigid body correction of head motion and changes in ventricles). Original data were smoothed in the spatial domain (Gaussian filter:FWHM=5mm). We used BrainVoyager and a customized Matlab code to calculate pairwise correlations between the average time-course signals, extracted from 246 ROIs defined by the Brainnetome atlas3 and$$$\,$$$34$$$\,$$$from suit4. All matrices were kept signed and unthresholded. Using connectome-based predictive modeling (CPM), we tested for predictive models of brain–behavior relationships from connectivity data using cross-validation5. This implies four consecutive steps: 1)$$$\,$$$feature selection, 2)$$$\,$$$feature summarization, 3)$$$\,$$$model building, and 4)$$$\,$$$assessment of prediction significance. This produces a generalizable model with as input brain connectivity data and that generates predictions of behavioral measures in novel subjects, accounting for a large amount of the variance in these measures. DI preprocessing pipeline included brain extraction6, denoising7, susceptibility artifact correction8 and Eddy-current and head-motion correction9. The diffusion tensor model from Dipy was used to generate fractional anisotropy (FA) maps10,11. FA maps were normalized in the MNI space and opened in BV where a whole brain correlation was made with mood repair. The resulting map was corrected at$$$\,$$$p<0.05$$$\,$$$for multiple comparison using cluster-size thresholding (size=370mm³).
Results
CPM revealed that mood repair scores could be predicted from rsfMRI FC during meditation in the meditator group. The correlation between the predicted and measured values of mood repair scores was$$$\,$$$0.37$$$\,$$$(p=0.009) (Figure$$$\,$$$1A). The prediction significance of this correlation, based on 2.000 permutations, resulted in a p value of 0.01. The highest degree node was in the right ventral agranular insula (Figure$$$\,$$$2). Within the group of meditators, the FC values of the right ventral agranular insula with amygdala, hippocampus, nucleus accumbens, precuneus, posterior cingulate cortex, and parieto-occipital sulcus were significantly higher during meditation as compared to the non-meditation resting-state condition. Likewise, a between-group comparison revealed significantly higher FC values of the right ventral agranular insula with the parieto-occipital sulcus, amygdala, hippocampus, precuneus and posterior cingulate cortex in meditators compared to control subjects.The whole brain correlation between FA values and mood repair revealed$$$\,$$$2$$$\,$$$clusters, one in the right uncinate fasciculus (r=0,475;$$$\,$$$P<0,00056;$$$\,$$$Figure$$$\,$$$1B), in a region connected to the ventral agranular insula, and a second cluster in the right superior longitudinal fasciculus. A group comparison revealed a non-significant trend for higher FA values in the uncinate fasciculus in meditators compared to controls$$$\,$$$(p=0,065).
Discussion
The present data reveal that mood repair, a psychological trait positively involved in cognitive emotion regulation and coping with negative life events, can be predicted by connectome-based modelling of fMRI FC during meditation. The highest degree node was found in the right ventral agranular insula. Within the group of meditators, the FC of this area with other brain areas involved in emotion regulation, memory and cognitive control was significantly higher during meditation as compared to rest. The DI data revealed the implication of the uncinate fasciculus in mood repair. The uncinate fasciculus is part of the limbic system linking the anterior temporal lobe with the inferior prefrontal cortex. This pathway matures slowly and continues to develop until the age of$$$\,$$$3012. Lesions to the uncinate fasciculus are linked to cognitive, socio-emotional, and behavioral difficulties13.Acknowledgements
Quentin Dessain is a research fellow of the Fonds de la Recherche Scientifique - FNRS of Belgium.References
1. Brandmeyer T, Delorme A, Wahbeh H. The neuroscience of meditation: classification, phenomenology, correlates, and mechanisms. Prog Brain Res. 2019;244:1-29.
2. Mayer JD, Salovey P, Caruso DR. Emotional Intelligence: Theory, Findings, and Implications. Psychological Inquiry, 2004;15,197-215
3. Fan, L., Li, H., Zhuo, J., Zhang, Y., Wang, J., Chen, L., Yang, Z., Chu, C., Xie, S., Laird, A.R., Fox, P.T., Eickhoff, S.B., Yu, C. & Jiang, T. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cerebral Cortex, 26 (8): 3508-3526,(2016).
4. Diedrichsen, J. (2006). A spatially unbiased atlas template of the human cerebellum. Neuroimage, 33, 1, p. 127-138.
5. Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017 Mar;12(3):506-518.
6. Otsu, N., 1979. A threshold selection method from gray-level histograms. IEEE transactions on systems, man, and cybernetics, 9(1), pp.62-66.
7. Veraart, J., Novikov, D.S., Christiaens, D., Ades-Aron, B., Sijbers, J. and Fieremans, E., 2016. Denoising of diffusion MRI using random matrix theory. Neuroimage, 142, pp.394-406.
8. Andersson, J.L., Skare, S. and Ashburner, J., 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 20(2), pp.870-888.
9. Andersson, J.L. and Sotiropoulos, S.N., 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, pp.1063-1078.
10. Basser, P.J., Mattiello, J. and LeBihan, D., 1994. MR diffusion tensor spectroscopy and imaging. Biophysical journal, 66(1), pp.259-267.7.
11. Garyfallidis, E., Brett, M., Amirbekian, B., Rokem, A., Van Der Walt, S., Descoteaux, M., Nimmo-Smith, I. and Dipy Contributors, 2014. Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics, 8, p.8.
12. Lebel, C; Walker, L; Leemans, A; Phillips, L; Beaulieu, C. (2008). "Microstructural maturation of the human brain from childhood to adulthood". NeuroImage. 40 (3): 1044–55.
13. Phan, KL; Orlichenko, A; Boyd, E; Angstadt, M; Coccaro, EF; Liberzon, I; Arfanakis, K (2009). "Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder". Biological Psychiatry. 66 (7): 691–4.
Figures
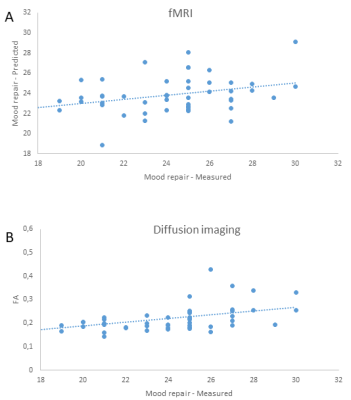
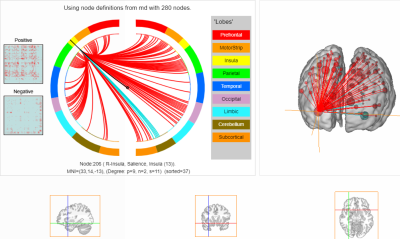
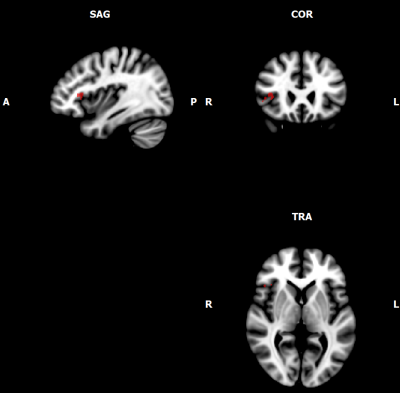
Figure 3: Area within the uncinate fasciculus correlating with measured mood repair scores (TMMS).