3496
Functional Connectivity in Smokeless Tobacco Dependent Users in a Craving ‘Primed’ State
Vasishta Polisetty1, S Senthil Kumaran2, Priyanka Bhat3, Pankaj .2, Anju Dhawan1, and Sonali Jhanjhee1
1Dept of Psychiatry, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 2Dept of NMR, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 3Biomedical Engineering, Indian Institute of Technology, Delhi, New Delhi, India
1Dept of Psychiatry, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 2Dept of NMR, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 3Biomedical Engineering, Indian Institute of Technology, Delhi, New Delhi, India
Synopsis
Keywords: Psychiatric Disorders, Brain Connectivity, Addiction
Tobacco is a leading cause of preventable cancer and death and is consumed in various forms. There is a paucity of literature regarding cue-induced craving in smokeless tobacco, commonly found in the South East Asian Region. This study aimed to investigate the functional connectivity in a ‘primed’ state after cue-induced craving. We found facilitatory connectivity within salience network (SN) and inhibitory connectivity between default mode network (DMN) and anterior cingulate cortex (ACC) in smokeless tobacco users.Introduction
Tobacco is a leading preventable cause of cancer and cardiovascular morbidity and mortality worldwide. The pattern of tobacco use varies widely, with the South East Asia Region posing a unique problem where a large proportion of tobacco is consumed in the smokeless variety.1 Understanding the neural network patterns underlying tobacco dependence can help devise better treatments for nicotine dependence by personalizing diagnosis, prognosis and therapeutics.2 Resting-state functional connectivity (rsFC) aids in understanding such dynamics across networks. We thus acquired rsFC to compare these patterns with that of healthy volunteers. The rsFC was acquired after ‘priming’ the subjects, by showing them the cues a few minutes before acquisition. Craving is a core symptom and criterion of substance dependence. Cue-induced craving is an emotional, motivational and cognitive response to conditioned cues. This study thus aimed to understand the rsFC smokeless tobacco-dependent users in a craving ‘primed’ state.Methods
Males (> 18 years), right handed were recruited in two groups: a) Cases [smokeless tobacco (ST), users for over 2 years as per ICD-10 nicotine dependence criteria]; b) Healthy Controls (never used ST).Any neuro-psychiatric comorbidity, any tobacco cessation treatment in the past 2 months, self-reported use of other substances in the past 72 hours, MRI contraindications and high-risk for other substances screened using WHO-ASSIST were criteria for exclusion for both groups. Subjects required a self-reported abstinence of a minimum of 2 hrs prior to imaging. Clinical variables pertaining to dependence (Fagerstrom Test for Nicotine Dependence - FTND), craving (Brief Questionnaire for Smoking Urges - BQSU), withdrawal (Minnesota Nicotine Withdrawal Inventory - MNWI) and motivation (Readiness to Change Questionnaire - RCQ) were evaluated before the scan.
Functional scans resting state were acquired using 3T MR scanner (Ingenia 3.0T, M/s. Philips HealthCare, The Netherlands) at dynamics = 300,TE/TR = 25/1000, acquisition matrix = 230x230x135, voxel = 3x3x3, slices = 45. A T1 sequence (for coregistration) and a cue-based paradigm (for priming) were acquired prior to resting state during which subjects focused at a fixation point on screen.
Analysis of functional data was done using Conn toolbox (21.a). Pre-processing included realignment, outlier detection (using ART-Artefact Detection Tool), co-registration (to T1), normalization (to MNI-Montreal neurological institute template) and smoothing (kernel of FWHM=8), denoising (band-pass filter= 0.008 to 0.09 Hz). At second-level analyses, contrast was defined to compare the connectivity of HC to ST. Independent t-tests were used.
Seed-based analysis was done with ACC as the seed, with a voxel threshold of p < 0.001 and cluster threshold of p < 0.001 corrected for FDR, with [T(30)>5.02]. Whole brain ROI(region of interest) to ROI connectivity (RRC) analysis was also done using a connectivity threshold of p < 0.001 and cluster threshold of p < 0.001 corrected for FDR.
Results
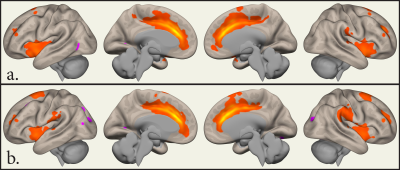
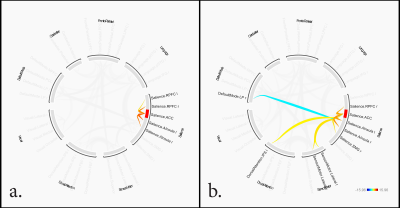
15 cases [age 39.7 ± 10.3] and 15 controls [age 32.6 ± 14.1]. Cases had a total duration of tobacco use of 21.0 ± 10.1 years (mean ± sd). FTND scores were 6.0 ± 1.4, indicating significant dependence and MWI scores 2.3 ± 2.4, indicating low scores on withdrawal. BQSU craving scores before imaging were 45.5 ± 15.8 and 49.4 ± 17.0 after imaging. There were 31.3% of the subjects in each of the pre-contemplation, contemplation and action stages of the RCQ.SBC revealed significant clusters of activation at ACC, bilateral para cingulate, and bilateral insular cortices in ST. These clusters were larger compared to that of HC (Figure 1). RRC in ST showed inhibitory network connectivity from ACC (of the Salience Network SN) to the right lateral Parietal (of the Default Mode Network DMN) and facilitatory network connectivity to the left Intraparietal sulcus (of the Dorsal Attention Network DAN) and bilateral lateral sensorimotor areas. Within the salience network ACC showed facilitatory connectivity to bilateral insular cortices, right supramarginal gyrus and right rostral prefrontal cortex (Figure 2).
Discussion
To our knowledge, this is the first study exploring rsFC (primed) in smokeless tobacco. Although connectivity has been studied in abstinence or during a cue paradigm; we performed a resting state analysis in a craving ‘primed’ state in acute abstinence, i.e. after a cue paradigm was administered. Increased coupling of the SN with the DMN in acute abstinence and while viewing a drug cue, along with a decoupling of the SN with the ECN has been reported.3–5 On the contrary, we observed that the ACC (SN) showed inhibitory connectivity to the DMN and facilitatory connectivity with the DAN and sensorimotor areas. These results could be explained by the subjects actively planning for drug use after the acute effects of craving. Unlike previous studies, in a ‘primed’ state the participant is not actively viewing the cue but is in anticipation of taking the drug once outside.Conclusion
A resting state connectivity in a primed state of craving has highlighted a facilitatory connectivity within salience network (SN) as well as between SN and anterior cingulate cortex (ACC) in smokeless tobacco users.Acknowledgements
No acknowledgement found.References
1. Sinha, D. Report on oral tobacco use and its implications in South-East Asia. SEARO WHO (2004).2. Ekhtiari, H., Nasseri, P., Yavari, F., Mokri, A. & Monterosso, J. Chapter 7 - Neuroscience of drug craving for addiction medicine: From circuits to therapies. in Progress in Brain Research (eds. Ekhtiari, H. & Paulus, M.) vol. 223 115–141 (Elsevier, 2016).
3. Maria, M. M. M.-S. et al. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict. Biol. 20, 407–414 (2015).
4. Claus, E. D., Blaine, S. K., Filbey, F. M., Mayer, A. R. & Hutchison, K. E. Association Between Nicotine Dependence Severity, BOLD Response to Smoking Cues, and Functional Connectivity. Neuropsychopharmacology 38, 2363–2372 (2013).
5. Brooks, S. J., Ipser, J. & Stein, D. J. Chapter 28 - Chronic and Acute Nicotine Exposure Versus Placebo in Smokers and Nonsmokers: A Systematic Review of Resting-State fMRI Studies. in Addictive Substances and Neurological Disease (eds. Watson, R. R. & Zibadi, S.) 319–338 (Academic Press, 2017). doi:10.1016/B978-0-12-805373-7.00028-1.
DOI: https://doi.org/10.58530/2023/3496