3479
Altered Glx and APT values in hippocampus of patients with aMCI: a novel combined imaging diagnostic marker1Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 2Liaocheng People’s Hospital, Liaocheng, China, 3Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China, 4Qilu Hospital of Shandong University, Jinan, China, 5Philips Healthcare, Beijing, China, 6The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 7F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Keywords: Alzheimer's Disease, Brain, Amnestic mild cognitive impairment; hippocampus; MEGA-PRESS; amide proton transfer-weighted imaging; imaging diagnostic marker
Amnestic mild cognitive impairment (aMCI) is considered as a prodromal stage of Alzheimer's disease (AD). Growing evidence supports the hypothesis that unbalanced excitatory/inhibitory (glutamate and gamma-aminobutyric acid) neurotransmitters and consequent abnormal protein deposition in hippocampus contribute to the pathological process of MCI and AD. We explored the changes of hippocampal Glx/GABA+ levels and APTw in aMCI patients using MEGA-PRESS and APTw imaging. Patients with aMCI exhibited decreased Glx levels (and Glx/GABA+ ratios) and increased APTw values in hippocampus. The combination of Glx and APTw values improved the diagnostic performance for aMCI, suggesting it was a potential imaging diagnostic marker.Introduction
Amnestic mild cognitive impairment (aMCI) is considered as a prodromal stage of Alzheimer's disease (AD). Growing evidence supports the hypothesis that unbalanced excitatory/inhibitory (glutamate and gamma-aminobutyric acid) neurotransmitters and consequent abnormal protein deposition in hippocampus contribute to the pathological process of MCI and AD, especially in the hippocampus. In this study, we aim to quantify hippocampal glutamate-glutamine (Glx) and gamma-aminobutyric acid (GABA)levels as well as amide proton transfer-weighted (APTw) signals of aMCI patients, and further to investigate the combined diagnostic performance of these metabolites.Methods
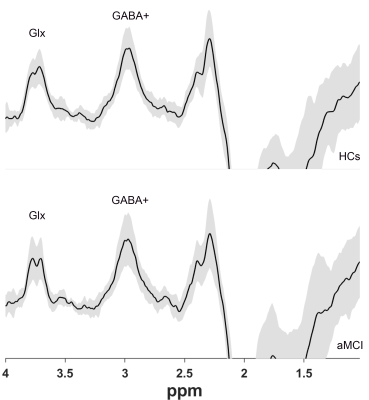
Twenty aMCI patients and 20 age- and gender-matched healthy controls (HCs) underwent MEGA Point Resolved Spectroscopy (MEGA-PRESS) and APTw MR imaging at 3 T prospectively. GABA+, Glx and APTw signals were measured in the right hippocampus. The GABA+ levels, Glx levels, Glx/GABA+ ratios and APTw values were compared between the aMCI and HC groups using Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was used to evaluate MEGA-PRESS and APTw parameters' diagnostic performance.Results
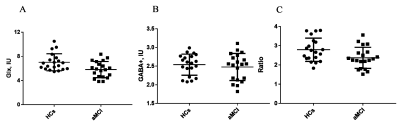
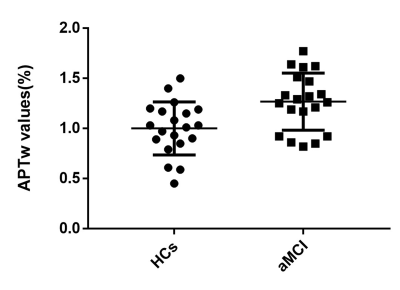
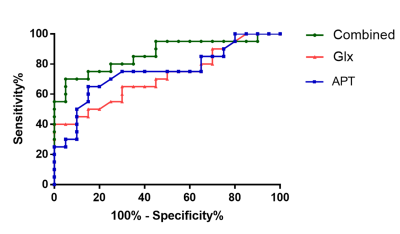
Glx levels were significantly lower in right hippocampus of patients with aMCI compared to HCs (7.02 ± 1.41 i.u. vs. 5.81 ± 1.33 i.u., P=0.018). No significant changes of GABA+ levels were observed in aMCI patients (HCs vs. aMCI: 2.54 ± 0.28 i.u. vs. 2.47 ± 0.36 i.u., P=0.620). In addition, Glx/GABA+ ratios between the two groups (HCs vs. aMCI: 2.79±0.60 vs. 2.37 ± 0.55, P=0.035) were significantly different. Compared with HCs, aMCI patients showed higher APTw values in the right hippocampus (1.26%±0.28 vs. 0.99±0.26%, P=0.006). ROC curve analysis showed the under the curve (AUC) of Glx and APTw values for diagnosing aMCI was 0.72 and 0.75 respectively, whilst the AUC value when combination of the two parameters increased to 0.86.Conclusion
Patients with aMCI exhibited decreased Glx level, Glx/GABA+ ratios and increased APTw values in right hippocampus. Combination of Glx levels and APTw values improved the diagnostic performance for aMCI, suggesting it as a promising combined imaging diagnostic marker.Discussion
Our study showed that patients with aMCI exhibit lower Glx levels and Glx/GABA+ ratios in the hippocampus than HCs, reflecting a potential hippocampal excitatory and inhibitory imbalance in aMCI patients. However, in this research, no significant changes of GABA+ levels were observed in aMCI patients, consistent with Huang’s study. According to the previous studies, glutamatergic neurotransmission in the hippocampus is severely disrupted in AD and was predominantly affected in the early stage of AD. Therefore, the changes of glutamatergic neurons may precede GABAergic neurons in E/I systems in the process of AD, and altered Glx level may be a significative marker for early diagnosis of MCI.Increased APTw values were found in aMCI patients, consistent with prior studies based on APTw imaging. Abnormal protein accumulation, such as extracellular amyloid plaques and intracellular neurofibrillary tangles, is a crucial pathological change of the MCI brain, and the accumulation of these abnormal proteins might contribute to the increase APTw signals. Therefore, APTw would be a promising imaging marker for the diagnosis of MCI.
No significant correlation was found between Glx levels and APTw values, indicating a promising complementary role between the two parameters for the diagnosis of MCI. Both Glx and APTw values have similar moderate diagnostic performance for aMCI. Combination of the two parameters demonstrated an increased AUC and improved the diagnostic performance, suggesting a potential imaging diagnostic strategy.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province [No. ZR2020QH268 (XC)].References
1. Mauri M, Sinforiani E, Zucchella C, Cuzzoni MG, Bono G. Progression to dementia in a population with amnestic mild cognitive impairment: clinical variables associated with conversion. Funct Neurol. 2012;27(1):49-54.
2. Bi D, Wen L, Wu Z, Shen Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer's disease. Alzheimers Dement. 2020;16(9):1312-29.
3. Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-705.
4. Canas PM, Simoes AP, Rodrigues RJ, Cunha RA. Predominant loss of glutamatergic terminal markers in a beta-amyloid peptide model of Alzheimer's disease. Neuropharmacology. 2014;76 Pt A:51-6.
5.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445-52.
Figures