3474
Rapid Frequency Offset Mapping with the Half-Fourier Acquisition Single-Shot Turbo Spin-Echo with the Selective Parity Approach1Radboud University, Nijmegen, Netherlands
Synopsis
Keywords: Quantitative Imaging, Brain
Frequency Offset maps are widely used to correct image distortion or estimate signal loss in gradient echo signal due to field inhomogeneities. Routinely, multiecho gradient echo sequences are used to collect this information. In this work, we introduce an innovative method which enables frequency offset mapping via one single-shot acquisition. The proposed method offers a significant reduction in the acquisition time without loss of accuracy.Introduction
Magnetic resonance imaging (MRI) can suffer from image distortion and signal loss due to the static magnetic field inhomogeneities. This is especially problematic in the case of fast MRI techniques like gradient echo imaging or echo planar readouts [1]. To correct distortion, frequency offset (FO) maps, also known as field maps [1-3], are often acquired.In this work, we propose the half-Fourier acquisition single shot spin-echo with the selective parity approach (SP-HASTE [4]) for a rapid FO mapping through only one single acquisition. This method offers several-folds of time saving which could be appealing for some applications such as MR thermometry or distortion-free B0 mapping.
Theory
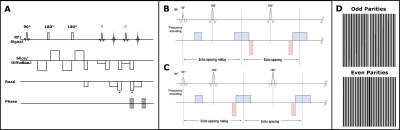
Diffusion-weighted (DW) SP-HASTE method (figure 1A) avoids image artefacts caused by anti-CPMG components, due to bulk motion in the presence of strong diffusion encoding gradients, through displacing gradients implemented before/after the readout gradients. This is achieved by moving the echo parity of less amplitude out of the acquisition window at each echo time, and hence, the method preserves the full sensitivity through benefiting from the contribution of both echo parities in the signal. On the contrary, the standard DW-HASTE method tackles the anti-CPMG condition through acquiring only one echo parity, halving the sensitivity to the signal.By imposing a delay time after the excitation radiofrequency pulse, and removing the diffusion encoding preparation module (figure 1B and C), we create an anti-CPMG condition with the difference that, here, any phase difference between the echo parities would be a direct result of the static magnetic field inhomogeneities, and not induced by bulk motion during the diffusion encoding. Acquired echo parities with SP-HASTE are then used to form 2 separate k-spaces (figure 1D). The odd and even k-space missing lines are later on estimated through the SPIRIT (GRAPPA) algorithm [5] to create 2 phase images for any delay time.
Methods
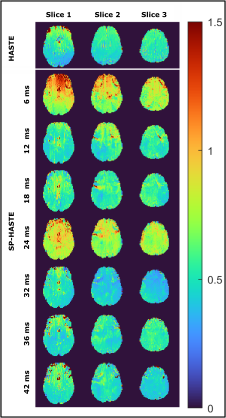
MRI scans were performed on a whole body 3T Siemens PrismaFit scanner (80 mT/m strength and 200 mT/m/ms slew rate) with a 32-channel receive-only head coil in 1 healthy female volunteer. HASTE, which requires several data acquisitions at different time points while increasing the imposed delay time after the excitation radiofrequency pulse, was used as our ground truth method. Common parameters were as follows: TR = 2000 ms, FOV= 200 * 200 mm, matrix size of 64*64, 3.1 mm isotropic in-plane resolution, 3 axial slices (3 mm), and 20 repetitions. No accelerated imaging was used for the data collection. Data were collected with delay times ranging from 0 to 42 ms with the increments of 6ms. Echo time and echo spacing were 50/7.34 ms for the HASTE and 13/7.09 ms for the SP-HASTE method, respectively.For the FO mapping, phase images were unwrapped using software distributed as part of the SEPIA toolbox [6]. For the SP-HASTE sequence, the 0ms delay time was disregarded since no phase shift is generated between the odd and even parity echoes. When using SP-HASTE method, only one acquisition was enough to generate the FO maps using:
$$FO = \frac{P_{even}-P_{odd}}{t}$$
where Podd and Peven are the odd and even parity phase images, respectively, and t is the delay time.
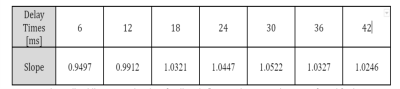
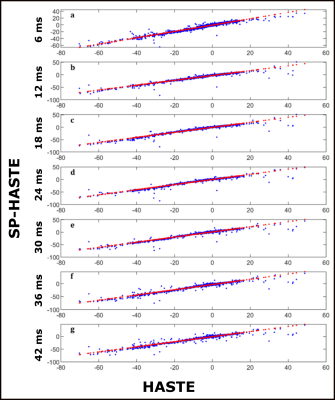
A linear regression was performed for the FO mean values (across the various repetitions) obtained with the SP-HASTE for each delay time versus the FO mean value from the HASTE method. To do so, the HASTE FO mean value was treated as the ground truth and slope and intercepts of a predicted line were estimated:
$$FO_{SP-HASTE, \triangle{T}}=b + m.FO_{GroundTruth}$$
where b is the intercept and m represents the regression slope.
The standard deviation (Std) was used as a measure of the image time course dispersion (noise). The temporal Std was calculated over 20 acquired repetitions for the FO maps obtained with both methods using:
$$σ=\sqrt{\frac{\sum_i^N (A_{i}-μ)^{2}}{N-1}}$$
where μ is the mean FO value of the 20 repetitions, N is the number of repetitions, and A represents the FO value within each voxel. For the SP-HASTE FO maps, the process was repeated for all the delay times.
Results
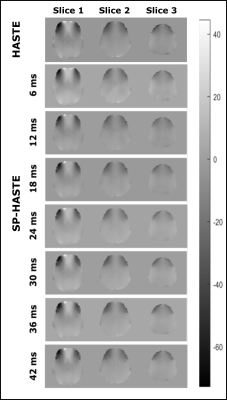
Created FO maps are presented in figure 2. The linear regression analysis results are shown in table 1 and figure 3. Calculated Std maps are presented in figure 4 for the FO maps obtained with HASTE and SP-HASTE methods. This shows the expected increase of SNR as of the FO estimation as the dephasing time approaches T2*, followed by a decrease due to reduced SNR.Discussion
The linear regression analysis performed on the estimated FO maps demonstrated a comparable agreement between the results from the SP-HASTE and HASTE methods for all the delay times with the slope of 1±0.05. The Std analysis worked as the quality evaluation of our measurements. The several-folds of time saving offered by the SP-HASTE method could find applications in generating distortion-free B0 maps and temperature mapping. The approach proposed here does not require the acquisition of dual-echo RARE, and will have a higher sensitivity than earlier approaches based on the separate acquisition of spin and stimulated-echo trains [7].Acknowledgements
Netherlands Organisation for Scientific Research (NWO) TTW-Grant 16313
Siemens Healthineers Nederlands
References
[1] J. J. Riera et al., “Effects of Field-Map Distortion Correction on Resting State Functional Connectivity MRI”, Frontiers in Neuroscience, 11, 656, 2017.
[2] R. Cusack, M. Brett, and K. Osswald, “An Evaluation of the Use of Magnetic Field Maps to Undistort Echo-Planar Images”, Neuroimage, 18(1), 127–142, 2003.
[3] P. Jezzard and R. S. Balaban, “Correction for geometric distortion in echo planar images from B0 field variations”, Magnetic Resonance in Medicine, 34(1), 65–73, 1995.
[4] A. Arbabi, V. Khlebnikov, J. P. Marques, and D. G. Norris, “Robust and Motion-Insensitive Approach to Diffusion-Weighted Half-Fourier Acquisition Single-Shot Turbo Spin-Echo Imaging”, ISMRM 2022.
[5] M. Lustig and J. M. Pauly, “SPIRiT: Iterative Self-consistent Parallel Imaging Reconstruction From Arbitrary k-Space”, Magnetic Resonance in Medicine, 64, 457–471, 2010.
[6] A. Karsa, K. Shmueli, “SEGUE: A Speedy rEgion-Growing Algorithm for Unwrapping Estimated Phase”, IEEE Trans Med Imaging, 38(6), 1347-1357, 2019.
[7] H. Paysen, K. Paul, M. Pham, L. Winter, and T. Niendorf, “Toward hybrid MR thermometry in aqueous and adipose tissue using simultaneous dual contrast weighting with double echo RARE imaging”, ISMRM 2017.
Figures