3470
Dedicated head-only high-performance gradients in a 3T scanner improve image quality compared to a whole-body gradient design1Walter Reed National Military Medical Center, Bethesda, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Fort Belvoir Community Hospital, Fort Belvoir, VA, United States, 4GE Global Research Center, Niskayuna, NY, United States
Synopsis
Keywords: Gradients, Brain
Ultra-high-performance MAGNUS gradient coils optimized for advanced diffusion imaging were used to obtain routine anatomical brain imaging in a cohort of 31 consecutive subjects enrolled in a mild TBI study. In a blinded review, three neuroradiologists reviewed 3D MPRAGE and 3D T2 FLAIR obtained with MAGNUS and a whole-body 3T scanner (WB3T). Substantial agreement of better overall image quality for MAGNUS vs. WB3T was seen for T2 FLAIR, and mild agreement of better overall image quality for MPRAGE. MAGNUS supports rapid gradient switching and higher gradient strength for additional SNR with 3D T2 FLAIR.
Introduction
MAGNUS (Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning) is a head-only ultra-high-performance gradient coil inserted into a clinical whole-body 3T MR system that can deliver simultaneous 200 mT/m and 500 T/m/s on each axis using a standard 1 MVA gradient driver1. MAGNUS peripheral nerve stimulation (PNS) threshold is higher than whole-body 3T MR (WB3T) scanners due to the smaller diameter and length of the gradient coil2. The MAGNUS higher PNS threshold allows up to 2.5x the slew rate of most WB3T scanners and ≥3-4x the maximum gradient amplitude1.Compared with typical WB3T gradient performance of 50 mT/m gradient amplitude and 200 T/m/s slew rate, we hypothesize that MAGNUS gradient performance would result in improved image quality of anatomical brain imaging in addition to the improved advanced diffusion imaging already demonstrated. Preliminary work with 3D T2 FLAIR brain imaging utilizing the MAGNUS higher gradient slew rate compared with WB3T resulted in decreased FSE echo spacing and improved SNR1. Similar improvements were found in anatomical imaging with a 25% reduction in 3D MPRAGE TR and a 20% reduction in TE with a 25% shorter fast gradient echo readout train after the magnetization preparation pulse1. This study aimed to compare two typical brain MR contrasts (3D T1 MPRAGE and 3D T2 FLAIR) obtained with the MAGNUS compared with WB3T in a cohort of subjects with acute mild traumatic brain injury (mTBI), chronic symptomatic mTBI, and normal controls.
Methods
Under an IRB-approved protocol enrolling mTBI subjects and age-matched normal controls, we selected a consecutive cohort of 31 subjects (13 men and 19 women, aged 18 to 57 years (mean 37.7, Q1 = 29, Q3 = 49) undergoing advanced diffusion MR and targeted anatomical brain MR imaging using the MAGNUS and WB3T on the same day. The specific parameters for the two sequences obtained on the MAGNUS and WB3T are provided in Table 1. The 3D T1 MPRAGE and 3D T2 FLAIR sequences on both the WB3T and MAGNUS used a self-calibrating data-driven parallel imaging acceleration3 factor of R = 2. We chose to use the optimized anatomical imaging parameters from the GE/NFL Head Health Initiative for our WB3T protocol4. We kept similar imaging time, acquisition matrix, FOV, and partition thickness on MAGNUS but acknowledge that the TR and TE on 3D MPRAGE will be shorter than WB3T due to improved gradient performance. We decreased bandwidth on MAGNUS 3D T2 FLAIR to keep the TE similar to WB3T.Three board-certified neuroradiologists individually reviewed the images in a blinded fashion on research PACS system (Horos v3.3.6) and viewed them using DICOM-compliant monitor (HP HC271). Pairwise comparison proceeded sequentially, with each neuroradiologist reviewing the images from one MR sequence in all 31 subjects before moving to the following MR sequence. The order in which the paired MAGNUS and WB3T were presented was randomized, with all identifying alphanumeric information removed. The neuroradiologists used a five-point Likert scale indicating a strong preference for the left image, preference for the left image, no preference, preference for the right image, and strong preference for the right image, as has been described previously5.
Statistical analysis: Three board-certified neuroradiologists reviewed 31 image sets for preference of overall image quality for the MAGNUS, WB3T, or equivalent. Ordinal ratings were assigned based on reviewers' image preference. The ranked coding was binarized for analysis in the reliability of agreement measure, Fleiss kappa, which is structured for multi-rater participation.
Results
MR imaging on both MR scanners was well tolerated in this consecutive cohort without severe PNS. There was excellent visualization of the entire posterior fossa and upper cervical cord on both scanners without image distortion or signal dropout. Cusp artifacts were present in 28 of 31 subjects scanned with MAGNUS, limited to the middle slices in the 3D sagittal volume, and did not encroach on the brain anatomy. Brighter intra-arterial signal was noted on MAGNUS 3D MPRAGE compared with WB3T. This has been described with other small gradient coil designs, which limit the saturation of incoming oxygenated blood.3D T2 FLAIR image rated by three neuroradiologists shows substantial agreement of better overall image quality for MAGNUS vs. WB3T (Fleiss k = 0.46). Overall agreement for rated review was 90.3% for 3D T2 FLAIR among raters. Figure 1 demonstrates a preference for MAGNUS 3D T2 FLAIR compared to WB3T.
3D MPRAGE image rated by three neuroradiologists shows mild agreement of better overall image quality for MAGNUS vs. WB3T (Fleiss k = 0.14), as illustrated in Figure 2. Overall agreement for rated review was 29% for MPRAGE among raters.
Future work will include blinded analysis of SNR, lesion conspicuity, gray/white contrast, cerebellar folia, and susceptibility artifact on MPRAGE, T2 FLAIR, SWI, and DWI.
Discussion and Conclusion
For standard clinical imaging with MAGNUS and WB3T protocol matched for imaging time and spatial resolution, we found a slight improvement in overall image quality on MPRAGE possible due to shorter TE and readout train with MAGNUS. The improvement in image quality was more striking for T2 FLAIR, where we could use the rapid gradient switching and higher gradient strength for additional SNR in terms of smaller bandwidth while matching the TR and TE of WB3T.Acknowledgements
The authors acknowledge the funding support from the U.S. Department of Defense, W81XWH-16-2-0054.
Disclaimer: The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University.
References
1. Foo TKF, Tan ET, Vermilyea ME, et al. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magn Reson Med 2020;83:2356–69.
2. Tan ET, Hua Y, Fiveland EW, et al. Peripheral nerve stimulation limits of a high amplitude and slew rate magnetic field gradient coil for neuroimaging. Magn Reson Med 2020;83:352–66.
3. Brau ACS, Beatty PJ, Skare S, et al. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med 2008;59:382–95.
4. Shetty T, Nguyen JT, Cogsil T, et al. Clinical Findings in a Multicenter MRI Study of Mild TBI. Front Neurol 2018;9:836.
5. Camerucci E, Campeau NG, Trzasko JD, et al. Improved Brain MR Imaging from a Compact, Lightweight 3T Scanner with High-Performance Gradients. J Magn Reson Imaging 2022;55:166–75.
Figures
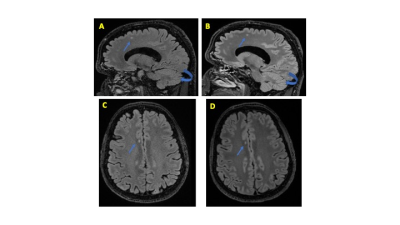
Figure 1. Comparison of 3D T2 FLAIR overall preference on whole-body 3T (WB3T) and Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning (MAGNUS)
Selected sagittal image of 3D T2 FLAIR obtained on the same day using the WB3T (A) and MAGNUS (B) with axial reconstruction through the centrum semiovale (WB3T – C, MAGNUS – D). All three reviewers preferred MAGNUS images. Notice the improvement in SNR, gray-white matter interface, lesion conspicuity (arrow), and cerebellar folia (curved arrow).
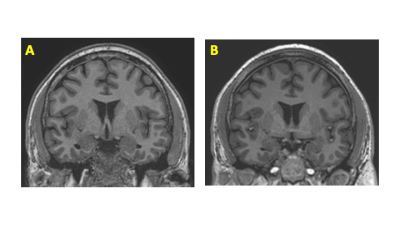
Figure 2. Comparison of 3D MPRAGE overall preference on whole-body 3T (WB3T) and Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning (MAGNUS)
Selected coronal reconstruction of the sagittal 3D MPRAGE through the centrum semiovale obtained on the same day using the WB3T (A) and MAGNUS (B). Two reviewers preferred MAGNUS images, and one had no preference. Notice the slight improvement in SNR in the deep white matter.