3461
Performance benchmark metrics and clinicopathologic outcomes of MRI-guided breast biopsies: a systematic review and meta-analysis
Berat Bersu Ozcan1, Justin Yan1,2, Yin Xi1, Serine Baydoun3, Marion E. Scoggins4, and Basak E. Dogan1
1Radiology, University of Texas Southwestern, Dallas, TX, United States, 2Radiology, HCA Houston Healthcare Kingwood, Houston, TX, United States, 3Radiology, Cleveland Clinic Foundation, Cleveland, OH, United States, 4Radiology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
1Radiology, University of Texas Southwestern, Dallas, TX, United States, 2Radiology, HCA Houston Healthcare Kingwood, Houston, TX, United States, 3Radiology, Cleveland Clinic Foundation, Cleveland, OH, United States, 4Radiology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Keywords: Breast, Breast, MRI-guided breast biopsy
MRI-guided breast biopsy (MRBB) methods and clinicopathological outcomes may vary between institutions. To identify benchmark metrics to help define a successful MRBB program, we identified and systematically reviewed articles on MRBB outcomes through 04.01.2021. Random intercept logistic regression model was used to pool the data. We found that MRBB is an efficient, highly accurate technique with high technical success (99.05%, 95%CI:97.75%-99.60%), low false-negative (0.65%, 95%CI:0.29%-1.44%), and complication (2.45%, 95%CI:2.13%-2.81%) rates. Our findings can assist the development of evidence-based clinical guidelines on follow-up recommendations in benign-concordant lesions and a transparent discussion with patients on the consequences of having an MRBB.Introduction
MRI-guided breast biopsy (MRBB) is a time-consuming and complex procedure that requires specific equipment and expertise. Current MRBB methods and subsequent clinicopathological outcomes may vary between institutions. We aimed to identify benchmark metrics to help define a successful MRBB program and guide institutional audits. To accomplish our goal, we identified and systematically reviewed studies to determine indications, technical success, histopathological outcome, false-negative, and upgrade rate of MRBB for institutional referencing.Methods
We identified studies involving MRBB through 04.01.2021 in the Embase database, Ovid Medline(R) Process, Other Non-indexed Citations, Ovid Medline(R), and completed a PRISMA checklist1. Inclusion criteria were English language literature, availability of reported histopathological outcomes of benign, malignant, and high-risk lesions, and availability of final histopathology (gold standard) or at least one imaging/clinical follow-up after biopsy. Sources of bias (QUADAS-2)2 were assessed. We tabulated numbers from all studies. Studies were excluded on a per-question basis when they did not report the numbers we were investigating. Random intercept logistic regression model was used to pool rates. Of note, the random effects model uses weighted proportions, so: 1) pooled rates are not calculated by dividing the nominator by the denominator, 2) denominators are different for each analysis, 3) pooled rates might not add up to 100%. Clopper-Pearson exact binomial intervals were calculated for each pooled proportion. Between-study heterogeneity was quantified by I2 statistic3. Odds ratios were pooled using random effects model.Results
Fifty-two papers with a total of 11,658 target lesions were retrieved (Fig.1). Varying magnet strength (1.5 or 3 Tesla), needle gauge (7-18), and types were used for biopsy. The number of patients was reported in 50/52(96.15%) studies and the mean patient age was 51.8 years (range of mean/median, 45.5-58, SD:±2.8) in 8,232 women. Twenty-seven studies out of 52(51.92%) provided the number of recommended biopsies along with the number of successful ones. The pooled rate for canceled biopsies due to non-enhancement on the day of the procedure was 5.28%(95%CI: 2.29%, 11.67%) (Fig.2.a.). Canceled biopsies due to non-enhancement were excluded from the technical success analysis, yielding a final technical success rate of 99.05%(95%CI: 97.75%, 99.60%) (Fig.2.b.). A total of 11,451 successful MRI-guided biopsies were included in the review. A median of 13 cores [range:2-60] was obtained per biopsy. MRI indication information was available in 5,585/8,232(67.84%) patients, and were breast cancer staging in 1,525(27.31%, 95%CI: 26.14%, 28.49%), screening in 1,468(26.29%, 95%CI: 25.13%, 27.46%), history of breast cancer in 1,027(18.39%, 95%CI: 17.38%, 19.43%), diagnostic in 1,038(18.59%, 95%CI: 17.57%, 19.63%), unknown primary in 74(1.33%, 95%CI: 1.04%, 1.66%), and other in 453(8.11%, 95%CI: 7.41%, 8.86%). Of MRI indications, less malignant outcomes were observed in screening (OR 0.47, 95%CI:0.25, 0.87; p=0.02) while no statistically significant difference was observed in other indications (Fig.3). Histopathology was benign in 65.33%(95%CI: 59.72%, 70.54%), malignant in 29.35%(95%CI: 23.60%, 35.84%) and high risk in 17.31%(95%CI: 10.12%, 28.02%). Overall, mass lesions were more likely to yield malignancy compared to non-mass and foci lesions [27.39% vs 11.36%(non-mass) and 18.03%(foci), p<0.001]. Surgical upgrade to invasive cancer occurred in 12.24% of DCIS (95%CI: 7.76%, 18.77%) and to malignancy in 15.58% of high-risk lesions (95%CI: 11.04%, 21.53%). ADH lesions upgraded to DCIS or invasive cancer in 31.13%(95%CI: 25.17%, 37.78%) while pooled upgrade rate of 6.75%(95%CI: 2.57%, 16.56%) was seen in high-risk lesions other than ADH (Fig.4). Among high-risk lesions, ADH had the highest upgrade rate to malignancy (OR 3.5, 95%CI: 2.18, 5.65, p<0.001]. MRI follow-up was performed in 1,651(20.05%) patients after benign results [median=25 months(range:0.4-117)]. Radiology-pathology discordance (2.48%, 95% CI: 1.62%, 3.77%) (Fig.5.a), false negative after a benign-concordant biopsy (0.65%, 95%CI: 0.29%, 1.45%) (Fig.5.b) and biopsy complications [seen in 202 out of 8,232 patients (2.45%, 95%CI: 2.13%, 2.81%); 169(1.48%) hematoma, 22(0.19%) vasovagal response, 19(0.17%) other] were rare.Discussion and Conclusion
MRI-guided breast biopsy is an efficient and highly accurate technique with high technical success and low false-negative and complication rates. We found a low false-negative rate in benign-concordant lesions which supports that there is no need to follow up patients with MRI after a benign-concordant biopsy result4-7. Our findings underscore that the surgical upgrade to malignancy is common among high-risk lesions, especially ADH. Traditionally, it was recommended to surgically excise high-risk lesions due to their high degree of underestimation on biopsy. However, the most recent recommendations advocate a more cautious multidisciplinary approach to assess the individual risk of patients and avoid surgical excision whenever possible8,9. Unfortunately, due to the lack of correlating data on patient history, we could not further investigate multivariable associations of surgical upgrade of high-risk lesions diagnosed at MRI-guided needle biopsy to predict individual risk of patients. Heterogeneity between groups and across studies and retrospective design (49/52, 94.2%) were limitations of our analysis.Our findings can be used to guide breast radiologist practice, inform transparent discussion with patients on the consequences of having an MRI-guided breast biopsy, and assist the development of evidence-based clinical guidelines on follow-up recommendations in benign-concordant breast lesions.
Acknowledgements
The authors have no conflicts of interest to declare. This work was supported by an internal grant by the University of Texas Southwestern, Simmons Cancer Center.References
1. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.2. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529-536.
3. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. Jun 15 2002;21(11):1539-58.
4. Pinkney DM, Chikarmane SA, Giess CS. Do benign-concordant breast MRI biopsy results require short interval follow-up imaging? Report of longitudinal study and review of the literature. Clin Imaging. Sep-Oct 2019;57:50-55.
5. Li J, Dershaw DD, Lee CH, Kaplan J, Morris EA. MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. AJR Am J Roentgenol. Sep 2009;193(3):850-5.
6. Huang ML, Speer M, Dogan BE, et al. Imaging-Concordant Benign MRI-Guided Vacuum-Assisted Breast Biopsy May Not Warrant MRI Follow-Up. AJR Am J Roentgenol. Apr 2017;208(4):916-922.
7. Lambert J, Steelandt T, Heywang-Köbrunner SH, et al. Long-term MRI-guided vacuum-assisted breast biopsy results of 600 single-center procedures. Eur Radiol. Jul 2021;31(7):4886-4897.
8. Rageth CJ, O’Flynn EA, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, Tausch C, Dammann F, Baltzer PA, Fallenberg EM, Foschini MP. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast cancer research and treatment. Apr 2019;174(2):279-96.
9. Pinder SE, Shaaban A, Deb R, Desai A, Gandhi A, Lee AH, Pain S, Wilkinson L, Sharma N. NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clinical radiology. Aug 2018;73(8):682-92.
Figures
Figure 1. Flow diagram of study selection process.*After exclusion of duplicates.
Note: Flow diagram template is taken from "Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.", as suggested in PRISMA 2020 guideline.

Figure 2. Forest plots and pooled estimates of a. the rate of the cancelled
biopsies due to non-enhancement on the day of the MRI-guided biopsy. b. the technical success rates in MRI-guided biopsies. * Cancelled biopsies due to non-enhancement on the day of biopsy were excluded from the technical success analysis. Recommended biopsy number reflects that exclusion. Prop., proportion, CI, confidence interval, I2,
I square, τ2, tau squared.
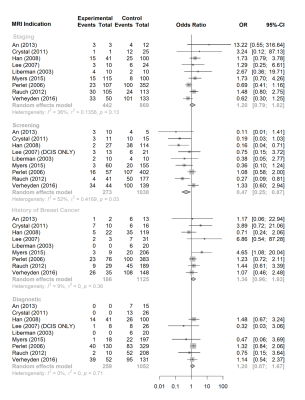
Figure 3. Forest plots showing the association
of MRI indication with the likelihood of malignancy outcome in MRI-guided
breast biopsy. Note: Included study results were homogenous in history of breast cancer,
staging and diagnostic indication groups whereas in screening group, they were
heterogenous (p values of random effects models: 0.36, 0.13, 0.71 and 0.03,
respectively). MRI, magnetic resonance
imaging; OR, odds ratio; CI, confidence interval, I2, I square, τ2, tau squared.
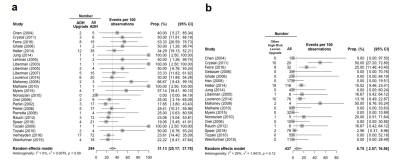
Figure 4. Forest plots and pooled estimates of a. upgrade rate of atypical ductal hyperplasia (ADH) b. upgrade rate of high-risk lesions other than ADH in MRI-guided breast biopsy. Note: Other high-risk lesions include, lobular carcinoma in-situ (LCIS), atypical lobular hyperplasia (ALH), flat epithelial atypical (FEA) radial scars (RSL)/complex sclerosing lesions (CSL). ADH, atypical ductal hyperplasia, prop.,
proportion, CI, confidence interval.

Figure 5. Forest plots and pooled estimates of a. radiology-pathology discordance rate after MRI-guided needle biopsy, b. malignancy identified* following a benign-concordant MRI-guided breast
biopsy (false negative). * Identified after follow-up (median, 25; range, 0.4-117 months) or immediate excision or re-biopsy. Prop., proportion, CI, confidence interval, I2,
I square, τ2, tau squared.
DOI: https://doi.org/10.58530/2023/3461