3457
Comparison of diffusion-weighted imaging ADC metrics for early prediction of neoadjuvant treatment response in HER2-negative breast cancer1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Radiology, University of Washington, Seattle, WA, United States, 3Radiology, University of Minnesota, Minneapolis, MN, United States, 4Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA, United States, 5I-SPY 2 Advocacy Group, San Francisco, CA, United States, 6Quantum Leap Healthcare, San Francisco, CA, United States
Synopsis
Keywords: Breast, Diffusion/other diffusion imaging techniques, treatment response prediction, biomarkers, neoadjuvant chemotherapy
In breast imaging, diffusion-based (DW) MRI delivers complementary quantitative value to conventional dynamic contrast enhanced MRI. ADC maps of tumor tissue calculated from DW-MRI, specifically mean ADC of the tumor region of interest, have shown promise for measuring early response to neoadjuvant chemotherapy (NAC). However, depending on intra-tumoral heterogeneity, other histogram-based ADC metrics may provide even better predictive performance than mean ADC, but are yet to be studied thoroughly. Here, we investigated nine ADC-based metrics regarding their predictive performance for NAC treatment response.
Background
The apparent diffusion coefficient (ADC) of tissue presents a biomarker that is sensitive to tumor cellularity; lower ADC values are measured in tumorous regions where water diffusion is restricted. ADC maps calculated from diffusion-weighted (DW) MRI have shown promise for measuring early response to neoadjuvant chemotherapy (NAC) of breast cancer, as presented in ACRIN 66981. These previous analyses identified tumor mean ADC as a marker to predict pathologic complete response (pCR). However, depending on intra-tumoral heterogeneity, other histogram-based ADC metrics may provide even better predictive performance than mean ADC, but are yet to be studied thoroughly. The objective of this work was to investigate the predictive power of histogram-based ADC metrics for early prediction (three weeks NAC) of pCR in HER2-negative (HER2-) breast cancer. The studied cohort includes patients receiving standard (‘control’) or experimental treatment.Methods
We retrospectively analyzed DW-MRI, dynamic contrast enhanced (DCE) MRI, and clinical outcomes in high-risk, stage II/III, HER2- female breast cancer patients (N=166). Patients underwent NAC with 12 weekly doses of paclitaxel (with or without an experimental agent), followed by 12 weeks of treatment with anthracycline. The analyzed cohort comprised the following HER2− patients: 86 patients with hormone-receptor [HR]+/HER2- subtype, and 80 patients with HR-/HER2- subtype. There were 70 (38 HR+/HER2-, 32 HR-/HER2-) patients who received the control agent only, and 96 patients (48 HR+/HER2-, 48 HR-/HER2-) who received one of four experimental drug agents (Pembrolizumab (N=25), MK2206 (N=17), Veliparib (N=37), or Neratinib (N=17)).DW- and DCE-MRI were performed with a dedicated breast coil according to the I-SPY2 scan protocol1,2 at pretreatment (T0) and after three weeks of NAC (T1). Data were obtained from 18 study sites utilizing multiple MRI vendors (Siemens, GE, Philips) and field strengths (1.5T, 3T). DW-MRI was acquired using a spin echo echo-planar imaging sequence with parallel imaging (R≥2) and fat suppression, and ADC maps were generated based on 2 b-values (0, 800 s/mm2).
MRI data were processed at our institution using dedicated in-house software developed in IDL (Exelis Visual Information Solutions) to calculate %-change from T0 to T1 in multiple metrics, defined as %-change = 100 × (MT1 – MT0)/MT0, where M denotes the metric of interest. For DW-MRI, tumor regions-of-interest (ROIs) were manually delineated by expert observers in ADC maps, focusing on diffusion-restricted regions3. Nine ROI-based ADC metrics were derived: mean, minimum, maximum, as well as 5th, 15th, 25th, 50th, 75th, and 95th percentiles. DCE-MRI was analyzed to calculate %-change in functional tumor volume (FTV, integral biomarker of I-SPY2) by considering signal enhancement ratio (SER) and percent enhancement (PE) using pre-, early-, and late-contrast data4. Clinical outcomes were classified at surgery as: pCR, treatment responders; non-pCR, treatment non-responders.
Predictive performance for pCR of each metric after three weeks of treatment (%-change from T0 to T1) was assessed by: the receiver-operating-characteristic (ROC) curve from a logistic regression model to predict pCR, the area-under-the-curve (AUC), and the Wilcoxon rank-sum test (p<0.05 was considered statistically significant).
Results
Fig. 1 lists results for the total cohort (pCR rate = 31.3%), HR+/HER2- only subgroup (pCR rate = 23.3%), and HR-/HER2- only subgroup (pCR rate = 40%). Fig. 2 plots corresponding ROC curves for all analyzed metrics.For every ADC metric, %-change from pretreatment to early treatment was higher in patients who reached pCR than non-pCR patients. Likewise, %-change in FTV was also stronger (more negative) for pCR than non-pCR patients. For the combined cohort (Fig. 2a), 75th percentile ADC was the most predictive diffusion-weighted MRI metric (AUC=0.64, 95% CI: [0.55, 0.71], p=0.005), though DCE-based FTV suggested slightly higher predictive performance (AUC=0.69, 95% CI: [0.62, 0.76], p<0.001).
For the HR+/HER- subgroup (Fig. 2b), mean ADC was the most predictive ADC metric (AUC=0.62, 95% CI: [0.48, 0.75], p=0.092), though estimated to have lower predictive performance than FTV (AUC=0.74, 95% CI: [0.62, 0.84], p=0.001), which was the highest AUC of all results. In the HR-/HER2- subgroup (Fig. 2c), both 75th percentile ADC and FTV yielded similar predictive performance (0.66, 95% CI: [0.55, 0.77], p=0.013 and 0.68, 95% CI: [0.62, 0.84], p=0.007, respectively).
Discussion and Conclusion
We investigated differences in the predictive performance for pCR of ADC in HER2- breast cancer patients receiving control or experimental drug agents. The data were analyzed with respect to the full dataset and also when stratified by hormone-receptor class (HR+ versus HR-).Though FTV suggested the highest predictive performance in either of the two subtypes, ADC metrics also showed acceptable performance and ADC percentile metrics suggested similar predictive performance to mean ADC. Further, results suggest that the performance of ADC-based metrics depends on HR class. This study did not attempt to perform an analysis stratified by drug arm, owing to small and imbalanced sample sizes for each experimental drug (n=17-37). We expect that ADC metrics become more predictive for respective single drug arm cohorts, as different drug arms yielded different pCR rates (here: 21.4%, 48%, 41.2%, 35.1%, 29.4% for control, Pembrolizumab, MK2206, Veliparib, and Neratinib, respectively). Thus, additional analyses with increased patient numbers for each experimental drug arm and cancer subtype are warranted to further investigate the utility of ADC-based metrics to complement FTV in breast NAC response monitoring.
Acknowledgements
This work was partially funded by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) grants R01 CA132870, U01 CA225427, P01 CA210961, and R01 CA255442.
References
1. Partridge et al., Radiology 289(3):618-27 (2018)
2. Li et al. J Magn Reason Imaging 50(6): 1742-53 (2019)
3. Nu et al., Tomography 8: 1208-20 (2022)
4. Newitt et al.,Transl Oncol 7(1):94-100 (2014)
Figures
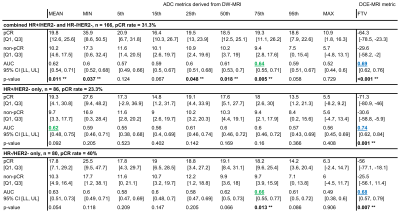
Figure 1. Analysis of %-change from pre- to early-treatment timepoints of nine DW-MRI ADC metrics and DCE-MRI FTV in (top) the combined HER- cohort, (middle) the HR+/HER2- only group, and (bottom) the HR-/HER2- only group. Row 1-4 in each block show median [Q1, Q3] of %-change over the respective patient group. The highest ADC-based AUC values are printed in green. pCR, treatment responders; non-pCR, treatment non-responders; [Q1, Q2], interquartile range; AUC, area-under-the-curve; CI [LL, UL], confidence interval; ** statistically significant p-values (p < 0.05).
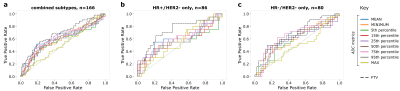
Figure 2. ROC curves describing the predictive performance of nine ADC metrics (solid, colored) and FTV (dashed, gray) in (a) the combined HER- cohort, (b) the HR+/HER2- only group, and (c) the HR-/HER2- only group.