3455
Diagnostic Value of Ultrafast Dynamic Contrast-Enhanced MRI in Assessing the Status of Axillary Lymph Node Metastasis in Breast cancer1The First Affiliated Hospital, Hengyang Medical School, University of South China, HengYang, China, 2MR Scientific Marketing, Siemens Healthineers Ltd, Wuhan, China, 3MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Keywords: Breast, Cancer
This study investigated the feasibility of ultra-fast DCE-MRI in accurately assessing axillary lymph node (ALN) metastasis in breast cancer. Our results showed that maximum slope (MS) accurately estimated the axillary lymph node status using the early-stage data of ultra-fast DCE-MRI. Furthermore, larger size (≥10mm diameter) of ALN and absence of hilum in the ultrafast DCE-MRI was associated with ALN metastasis in breast cancer patients. Our study demonstrated that MS from ultrafast breast MRI is a potential imaging biomarker of ALN metastasis in breast cancer.
Introduction
Axillary lymph node metastasis (ALNM) in breast cancer (BC) patients is associated poor prognosis1-2.Therefore, accurate evaluation of ALNM status is necessary to select appropriate treatment strategy that improves survival outcomes3.Currently, axillary lymph node (ALN) status in BC patients is diagnosed using invasive methods, such as, sentinel lymph node biopsy (SLNB) and ALN dissection4-6.This study aimed to evaluate the clinical value of ultrafast dynamic contrast enhanced (DCE) MRI in the differential diagnosis of metastatic and non-metastatic ALNs in BC.Methods
This prospective study was approved by the Institutional Review Board of our hospital. Forty-nine women with breast cancer were recruited between April 2022 and October 2022. Finally, 36 BC patients were included after applying the exclusion criteria (Figure 1). The breast mass and axillary lymph node status was confirmed by histopathology.All the patients underwent pre-operative breast MRI examinations in a 3T MR scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany). DCE and T1 map were also performed using TWIST-DIXION-VIBE and T1 VIBE sequences for semi-quantitative analysis, respectively. The imaging parameters for the T1 maps were as follows: TR/TE, 6.20/2.71 ms; spatial resolution, 1.6 × 1.6 × 2.0 mm3; matrix, 154×192; flip angle, 10° and 2°; FOV, 300×300 mm2; slice thickness, 2 mm. The imaging parameters for DCE were as follows: TR/TE, 3.9/1.23 ms; spatial resolution, 1.8 × 1.8 × 3.0 mm3; matrix, 97×192; flip angle, 9°; FOV, 340×191.3 mm2; CAIPIRINHA acceleration factor, 4; 4.14 s per phase; total scan time, 90.3 s. Early semi-quantitative kinetic parameters such as time to enhancement (TTE) and maximum slope (MS) were analyzed using the prototype research application MR DCE version 1.1.0 (Siemens Healthcare, Erlangen, Germany).
Statistical analysis was performed using the SPSS 22.0 software (SPSS, IBM, Somers, NY). The qualitative variables between metastatic and non-metastatic ALNs were compared using the Chi-square test. The differences in the TTE and MS estimates between metastatic and non-metastatic ALNs were analyzed using the independent sample t-test and Mann-Whitney U test, respectively. The receiver operating characteristic (ROC) curve analysis was performed to evaluate the performance of MS in predicting the metastatic ALN status. P<0.05 was considered statistically significant.
Results
DCE-based Morphological Parameters Show Significant Differences Between Metastatic and Non-Metastatic ALNs in BC PatientsHistopathologic examinations showed 19 (52.77%) metastatic ALNs and 17 (47.22%) non-metastatic ALNs. The differences in various morphological and kinetic parameters variables between the metastatic and non-metastatic ALNs are listed in Table 1. A higher proportion of metastatic ALNs showed larger diameter (≥10mm) and absence of hilum compared to the non-metastatic ALNs (P<0.05). The predictive performances of parameters such as ALN diameter (larger versus smaller) (sensitivity, 0.647; specificity,0.737; accuracy,0.694) and hilum (presence versus absence) (sensitivity,0.765; specificity, 0.632; accuracy, 0.694) were moderate for differentiating between metastatic and non-metastatic ALNs (Table 2).
MS shows superior diagnostic performance in distinguishing between metastatic and non-metastatic ALNs
MS values were significantly higher in patients with metastatic ALNs than those with non-metastatic ALNs (P<0.001), but the TTE values were comparable between the two groups (Table 1). Figures 2 and 3 show the representative DCE images and the TTE and MS maps of breast cancer patients with metastatic and non-metastatic ALNs. The area under the ROC curve (AUC) value for MS in discriminating between metastatic and non-metastatic ALNs was 0.858 (standard error, 0.06; 95% CI, 0.734–0.981; p < 0.001) (Figure 4).
Discussion
MS, the semiquantitative kinetic parameter derived from ultra-fast DCE-MRI data, showed superior performance to other morphological parameters in the preoperative diagnosis of ALN metastasis in BC patients.Ultra-fast DCE-MRI can detect early tumor angiogenesis and changes in vascular permeability based on semi-quantitative parameters such as MS and TTE, which are directly related to the underlying physiological properties7. MS reflects tumor perfusion and early contrast agent leakage from the blood vessels into the extravascular extracellular space, whereas TTE reflects vascular permeability8-9. Our study showed that MS values in BC patients with metastatic ALNs were significantly higher than those in the non-metastatic group. The enhancement rate was higher in the metastatic lymph nodes because of increased vasculature and faster perfusion rate.
The size of lymph nodes and the presence or absence of hilum are useful criteria for predicting the degree of lymph node involvement in BC10. In our study, larger size of the ALNs and absence of hilum were useful indexes for predicting ALN metastasis in patients with BC. This was consistent with findings from previous studies11. However, the prediction accuracy of MS was superior to the morphological parameters.
Our study suggested that prediction of nodal status in BC according to morphological parameters alone was not accurate and required improvement. Zhang et al 12 reported that the diameter of metastatic nodes was shorter than that of non-metastatic nodes. This was contradictory to our findings in this study. We postulate that a smaller sample size in our study may account for this discrepancy.
Conclusion
Ultrafast DCE-MRI shows good diagnostic performance in differentiating between metastatic and non-metastatic ALNs in BC. MS is a potential imaging biomarker for assessing ALN metastasis in BC patients.Acknowledgements
No acknowledgement found.References
1. Barkur S, Notingher I, Rakha E. Intra-operative assessment of sentinel lymph nodes for breast cancer surgery: An update[J]. Surgical Oncology, 2022, 40: 101678.
2. Sivridis, E.; Giatromanolaki, A.; Galazios, G.; Koukourakis, M.I. Node-Related Factors and Survival in Node-Positive Breast Carcinomas. Breast Edinb. Scotl. 2006, 15, 382–389.
3. Shaitelman SF , Cromwell KD, Rasmussen JC, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 2015;65(1):55–81.
4. Soares, E.W.S.; Nagai, H.M.; Bredt, L.C.; da Cunha, A.D.; Andrade, R.J.; Soares, G.V.S. Morbidity after Conventional Dissection of Axillary Lymph Nodes in Breast Cancer Patients. World J. Surg. Oncol. 2014, 12, 67.
5. Belmonte, R.; Garin, O.; Segura, M.; Pont, A.; Escalada, F.; Ferrer, M. Quality-of-Life Impact of Sentinel Lymph Node Biopsy versus Axillary Lymph Node Dissection in Breast Cancer Patients. Value Health 2012, 15, 907–915.
6. Andersson Y, Frisell J, Sylvan M, de Boniface J, Bergkvist L. Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. J Clin Oncol 2010;28(17):2868–2873.
7. rouge C, Guinebretière JM, Contesso G, Di Paola R, Bléry M. Correlation between contrast enhancement in dynamic magnetic resonance imaging of the breast and tumor angiogenesis. Invest Radiol 1994; 29:1043–1049
8. Taylor JS, Reddick WE. Evolution from empirical dynamic contrast-enhanced magnetic resonance imaging to pharmacokinetic MRI. Adv Drug Deliv Rev 2000; 41:91–110
9. Cuenod CA, Balvay D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 2013; 94:1187–1204
10. Rautiainen S, Masarwah A, Sudah M, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 2013;269(1):54–60.
11. Nahas SC, Nahas CSR, Cama GM, et al. Diagnostic performance of magnetic resonance to assess treatment response after neoadjuvant therapy in patients with locally advanced rectal cancer. Abdominal Radiol. 2019;44(11):3632–3640.
12. Zhang X, Zheng C, Yang Z, et al. Axillary sentinel lymph nodes in breast cancer: quantitative evaluation at dual-energy CT[J]. Radiology, 2018, 289(2): 337-346.
Figures
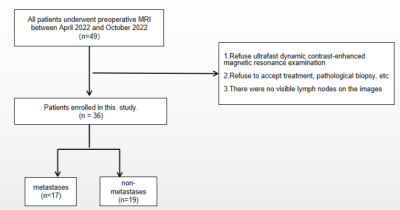
Figure 1. Flow chart of patient selection strategy.
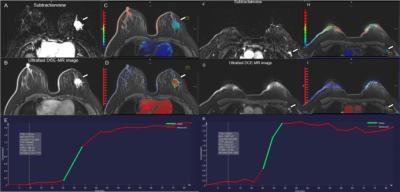
Figure 2. A 36-year-old woman with invasive ductal carcinoma in the left breast with left axillary lymph node metastasis. The irregular heterogeneous enhancing mass and lymph node metastasis in the left breast is shown in the (A, F) early phase MR subtraction images, (B, G) ultrafast DCE images, (C, H) time to enhancement (TTE) maps (C, 17.62s; H, 14.44s), (D, I) maximum slope (MS) maps (D, 6.7%/s; I, 12.9%/s), and (E, K) represent the early time-intensity curves of breast tumor and lymph node, respectively.
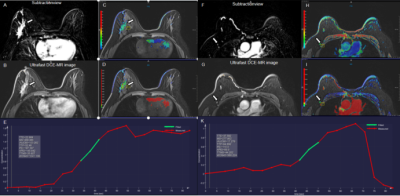
Figure 3. A 61-year-old woman with high-grade ductal carcinoma in the right breast without axillary lymph node metastasis. An irregular heterogeneous enhancing mass and a lymph node in the right breast is shown in the (A, F) early phase MR subtraction images, (B, G), ultrafast DCE images, (C, H) time to enhancement (TTE) maps (C, 22.96s; H, 37.58s), (D, I) maximum slope (MS) maps (D, 4.6%/s; I, 2.8%/s), and (E,K) represent the early time-intensity curves of breast tumor and lymph node, respectively.
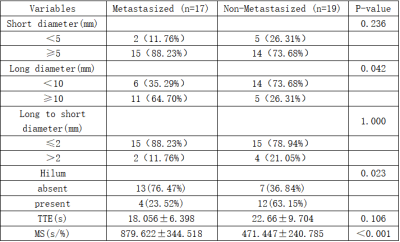
Table 1. DCE-MRI Derived Morphological and Kinetic Characteristics of Non-Metastatic and Metastatic ALNs in Breast Cancer Patients
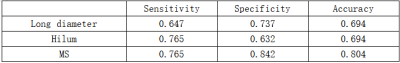
Table 2. Diagnostic performances of morphological parameters and MS in discriminating between metastatic and non-metastatic sentinel lymph nodes.
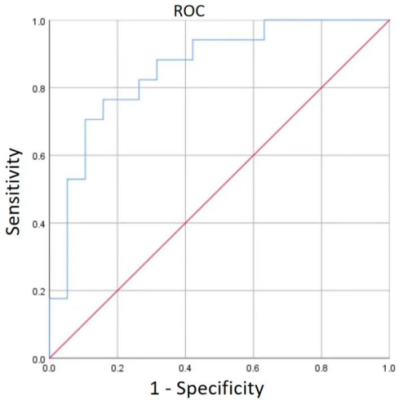
Figure 4. ROC curves show the predictive performance of MS in discriminating between metastatic and non-metastatic ALNs of patients with breast cancer.