3447
Amide proton transfer weighted (APTw) imaging-based radiomics in the molecular subtypes prediction and grading of adult-type diffuse gliomas1Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, beijing, China, 2Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, Hangzhou, China, 3Beijing Tiantan Hospital, Capital Medical University, Beijing, China, beijing, China, 4MR Collaboration, Siemens Healthineers Ltd., Beijing, China, beijing, China, 5College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, Hangzhou, China
Synopsis
Keywords: Radiomics, Brain, amide proton transfer weighted (APTw) imaging
The fifth edition of the 2021 WHO Classification of Tumors of the Central Nervous System (WHO CNS5) has introduced significant changes in classifying glioma subtypes based on molecular profiles. This study investigated the feasibility of amide proton transfer weighted (APTw)-based radiomics for predicting molecular subtypes and WHO grade of adult-type diffuse gliomas. APTw-based radiomics achieved satisfactory performance in distinguishing three molecular subtypes and WHO grade (High (Ⅳ grade)/Low (Ⅱ-Ⅲ grade)). This application may be an effective and promising quantitative approach for better noninvasive characterization and classification of gliomas.Introduction and Purpose
Adult-type diffuse gliomas can be classified into three molecular subtypes (IDH-mutant/1p/19q-codeleted oligodendrogliomas; IDH-mutant astrocytomas; IDH-wildtype glioblastomas) based on the status of IDH mutation and 1p/19q codeletion (1). Accurate glioma grading and molecular subtypes prediction are critically essential for individualized preoperative treatment decisions. There are a few studies that reported molecular subtypes are associated with biophysical parameters detected by MRI, particularly based on advanced imaging techniques at high field, such as amide proton transfer weighted (APTw) imaging (2-5). APTw imaging is a molecular MRI technique that generates image contrast based mainly on the amide protons in endogenous mobile cellular proteins and peptides in tissue (6). APTw MRI has the potential to detect IDH mutation status and to differentiate high-grade from low-grade gliomas (2-5) in a single MRI scan. Nevertheless, most of the previous works using limited number of cases only focused on the prediction of single IDH alteration. Radiomics is a rapidly evolving field of research concerned with the extraction of abundant quantitative metrics-the so-called radiomic features-within medical images, which could capture tissue and lesion characteristics that can be used for clinical problem solving (7). This study aimed to evaluate the utility of radiomics based on APTw imaging for predicting molecular subtypes and WHO grade (High (Ⅳ grade)/Low (Ⅱ-Ⅲ grade).Materials and Methods
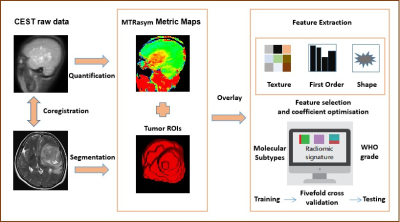
We prospectively collected 129 patients with adult-type diffuse gliomas (29 IDH-mutant/1p/19q-codeleted oligodendrogliomas; 59 IDH-mutant astrocytomas; 41 IDH-wildtype glioblastomas) (Table1). All patients received conventional MRI scan and APTw imaging on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). The main acquisition parameters for whole-brain CEST imaging (8) were as follows: RF saturation power/duration = 2.5uT/1sec, TR/TE = 3000/17ms, FOV = 212x212mm2, slice thickness = 2.79mm, and 7 frequency offsets including an unsaturated frame and 6 saturated frames (±3ppm, ±4ppm, and ±3.5ppm), and total acquisition duration = 4.6min. In addition, a dual-echo gradient-echo sequence with TE = 4.92/9.84ms was deployed for B0 field mapping. Conventional T1-weighted images (TR/TE = 1560/1.65ms, slice thickness = 1mm) and T2-weighted images (TR/TE = 5020/105ms, slice thickness = 5mm) were also acquired for defining the regions of interest (ROI). APTw images were processed by quantification of the magnetization transfer ratio (MTR = 1-Ssat/S0) asymmetry (MTRasym) analysis with respect to the water resonance. Cases were randomly assigned to the training and testing cohort at a ratio of 7:3. After feature extraction from the APTw images by FAEv0.5.2 software (9), different radiomic models are trained with stratifed fivefold cross validation to determine molecular subtypes and WHO grade. Flowchart of the whole process was showed in Figure 1. Accuracy and area under the curve (AUC) were determined to evaluate the performance of these radiomic models.Results
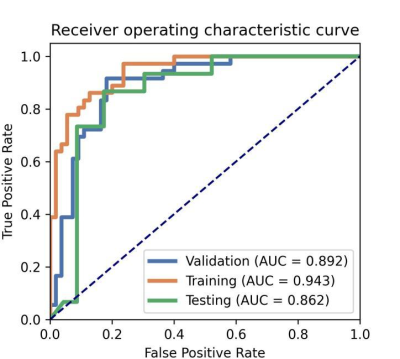
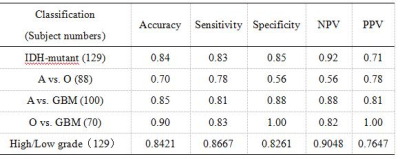
Radiomics model-1 (IDH genotype prediction) achieves accuracy of 89% (AUC = 0.9231) in the fivefold cross validation and 84% (AUC = 0.87) in the testing cohort (Figure 2A, Table 2). Radiomics model-2 (1p/19q genotype prediction from IDH-mutant giloma) achieves poor accuracy of 63% (AUC = 0.63) in the testing cohort (Figure 2B, Table 2). Radiomics model-3 and 4 have satisfactory performance in distinguishing glioblastomas from astrocytomas or oligodendrogliomas, achieving accuracies of 85% (AUC = 0.86) and 90% (AUC = 0.97) in the testing cohort, respectively (Figure 2C and D, Table 2). Radiomics model-5 (WHO grade High (Ⅳ grade)/Low (Ⅱ-Ⅲ grade)) achieves accuracy of 84% (AUC = 0.86) in the testing cohort (Figure 3, Table 2). The fivefold cross validations show a comparable predictive performance with that on the testing cohort, suggesting the efficacy of the trained classifiers Figure 2 and 3.Discussion
Using a relatively large dataset, we developed five APTw-based radiomics models to distinguish molecular subtypes and WHO grade of adult-type diffuse gliomas. The most important findings are that APTw-based radiomics show satisfactory performance in distinguishing IDH genotype and High (Ⅳ grade)/Low (Ⅱ-Ⅲ grade). However, the APTw-based radiomic for 1p/19q genotype prediction from IDH-mutant giloma show disappointing performance, which indicate APTw signal is associated with the malignant degree of glioma. Taken together, these results suggest that APTw signal are potential imaging biomarkers for preoperative early evaluation of virtual molecular pathology for patients with glioma.Conclusion
Our results indicate that the use of radiomics based on APTw imaging is feasible and can achieve high accuracy in differentiation of three molecular subtypes and WHO grade in glioma.Acknowledgements
No acknowledgement found.References
[1] Louis, D.N., Perry, A., Wesseling, P., et al., The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021; 23(8): 1231-1251.
[2] Jiang S, Eberhart CG, Zhang Y, et al. Amide proton transfer-weighted MR image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur J Cancer 2017;83:9–18.
[3] Zhou J, Zhu H, Lim M, et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 2013;38:1119–28.
[4] Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 2014;16:441–8.
[5] Jiang S, Zou T, Eberhart CG, et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn Reson Med 2017;78:1100–9.
[6] Zhou J, Heo HY, Knutsson L, van Zijl PCM, Jiang S. APT-weighted MRI: techniques, current neuro applications, and challenging issues. J Magn Reson Imag2019;50(2):347e64.
[7] Mayerhoefer ME, Materka A, Langs G, Häggström I, Szczypiński P, Gibbs P, Cook G. Introduction to Radiomics. J Nucl Med. 2020 Apr;61(4):488-495.
[8] Zhang Y, Yong X, Liu R, et al. Whole-brain chemical exchange saturation transfer imaging with optimized turbo spin echo readout. Magnetic Resonance in Medicine 2020;84(3):1161-1172.
[9] Y. Song, J. Zhang, Y. Zhang, Y. Hou, X. Yan, Y. Wang, M. Zhou, Y. Yao, G. Yang. FeAture Explorer (FAE): A tool for developing and comparing radiomics models. PLoS One. 2020;15(8):e0237587.
Figures