3432
Glutamate Predicts Post-Concussion Symptoms in Collegiate Athletes1Brigham and Women’s Hospital, Boston, MA, United States, 2University of Michigan, Ann Arbor, MI, United States, 3Ludwig-Maximilians-Universitat, Munich, Germany, 4University of Colorado-Anschutz, Aurora, CO, United States, 5Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Data Analysis, Contrast Mechanisms, Traumatic Brain Injury
A population of collegiate athletes, along with matched age and gender-matched student-athlete controls, were evaluated post-concussion, for neurometabolic levels in the PCG by magnetic resonance spectroscopy (MRS) and Post-Concussion Symptom Scale (PCSS) following injury. Evaluations were conducted 72 hours post-diagnosis. For the purpose of this work, correlations between total Glutamate (Glu+Gln), total Glutamate to total Creatine ratios, and symptoms on the PCS scale associated with cognitive fatigue - such as mental fog, drowsiness, fatigue, and feeling slowed - were explored.Introduction
Sport-related Concussion (SRC) is a common occurrence in sports with over 10,000 SRC occurring in collegiate athletes annually1. In the absence of an objective test for diagnosis, clinicians rely on patient-reported symptoms to determine diagnosis2. These clinical symptoms are usually measured using the Post-Concussion Symptom Scale (PCSS), which has been incorporated into commonly used concussion assessment tools like the Sports Concussion Assessment Tool (SCAT5) and the Immediate Post-Concussion Assessment and Cognitive Test (IMPACT). Of the symptoms evaluated, the most frequently reported are fatigue/low energy, drowsiness, and difficulty concentrating3. This abstract aimed to evaluate any potential link between brain metabolites and the presentation of clinical symptoms of fatigue following concussion in collegiate student-athletes. It was hypothesized that differences in brain metabolites would correlate with increased reported symptoms of brain fog and fatigue post-concussion.Methods
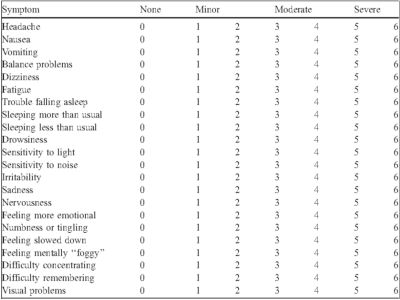
Subjects: 53 subjects, including the controls (n=22), were varsity collegiate athletes at a single NCAA Division 1 institution who consented to participate in the study. They underwent a clinical test and MRS scan following the diagnosis of a concussion. Control participants were age, sport, and gender-matched student-athletes who did not have a history of concussion or other head trauma within the past year.Clinical Measures: The Post-Concussion Symptom Scale (PCSS) was used to measure clinical symptom burden following concussion. Subjects were given a list of 22 symptoms shown in Figure 2 and asked to rate each symptom on a scale that ranges from 0 (asymptomatic) to 6 (severe). Subjects were instructed to rate only those symptoms that had started following the onset of the injury. The 22 symptoms were then further subdivided into five symptom scale domains: somatic, cognitive, sleep, emotional, and vestibular-ocular (vo).
Data collection: The study presents an analysis of spectra from each participant measured at a single brain location, the Posterior Cingulate Gyrus (PCG) selected for its sensitivity to brain injury. The scans were performed at 3T (Skyra, Siemens, Erlangen, Germany) using PRESS localization and utilizing the following parameters. TR=2000, TE=30ms, voxel size=20x20x20mm 3, number of averages=128.
Data Processing and Quantification: MRS datasets were processed with a python pipeline using OpenMRSLab 6 and quantified with LCModel7. This pipeline completed coil combination, eddy-current correction, frequency alignment, water removal, and zero-order phase removal. A TE correction factor was also applied to all absolute metabolite measurements.
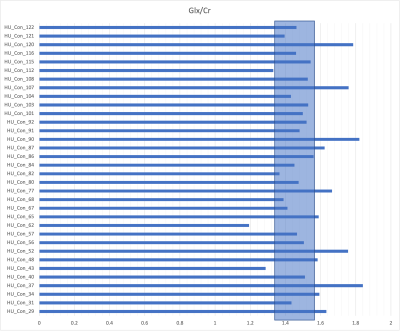
Statistical Methods: Normal ranges for the Glutamate and Glutamine (Glx) concentration and ratio to total Creatine (Cr+Pcr) were determined based on the controls of the study. The categories were sorted into “low” (1 standard deviation (stdev) > mean), “high” (1 stdev > mean), or “normal” (within 1 stdev of mean). These values were then used to sort the data collected from the concussed patients (n=35). Symptom scores were also sorted into “normal”(<3) and “high”(≥3) categories. Chi-squared tests of independence were then performed to compare each symptom to the metabolite ratio.
Results
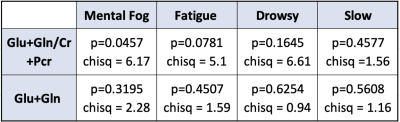
Of the 4 symptom scores that were compared to neurometabolic ratios in the PCG region of the brain, one was found to be statistically significant at p<.05. The correlation between Glx/Cr and mental fog symptom scores among concussed patients was significantly different than the expected distribution (p=.0457, chi-square = 6.17). Drowsiness, fatigue, and feeling slowed down had no significant deviation from the expected distribution (p=.1645, chi sqr = 6.61; p=.0781, chi sqr = 5.1; p=.4577, chi sqr =1.56). There was no significant correlation between the burden of the symptoms and the concentrations of Glx.Discussion
The data indicates that the Glx/Cr ratio in the PCG region correlates to mental fog post-concussion. Glutamate is the primary excitatory neurotransmitter of the central nervous system and is commonly used as a marker for the health of neuron-astrocyte interaction. Excessive and prolonged excitatory glutamatergic stimulation leads to neuronal cell death. An increase in glutamate and glutamine (Glx) has been associated with changes in clinical behaviors. In a previous study, it was found that an increase in Glx had a strong correlation with higher reported levels of cognitive fatigue1. Previous studies have shown that concussed individuals have higher Glx/Cr ratios and it has been noted that there is an excess release of Glx following traumatic brain injuries5,6. Concussed individuals are both more likely to have elevated ratios of Glx and more feelings of mental fog. The other three symptoms looked at are often reported by patients who also experience mental fog, however, they were not shown to have a significant correlation to Glx/Cr in this study.Conclusion
The sample size of this study was small and from a single institution which limits its ability to be generalized to larger samples. There is a possible link between Glx and clinical symptom presentation following concussion but larger datasets would be needed to confirm the response. Additionally, these changes were only observed in one region of the brain. It is possible that if other regions are explored or if the sample size is expanded, more significant deviations from control patients would be found. Other studies have explored how therapeutics could modulate Glx. If the findings of this work remain true for larger samples, it is possible that concussion symptoms could be reduced with drug interventions.Acknowledgements
No acknowledgement found.References
1. Zuckerman S L, et al. Epidemiology of Sport Related Concussion in NCAA Athletes From 2009-2010 to 2013-2014: Incidence, Recurrence, and Mechanisms. AJSM. (2015).
2. McCrory P, et al. Consensus Statement on Concussion in Sport – the 5th International Conference on Concussion in Sport Held in Berlin, October 2016. BJSM. (2017).
3. Shehata N, et al. Sport concussion assessment tool: baseline values for varsity collision sport athletes. BJSM. (2009)
4. Provencher SW, Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. (1993).
5. Kierans AS, et al. Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology. (2014)
6. Sowers JL, et al. Traumatic brain injury induces region-specific glutamate metabolism changes as measured by multiple mass spectrometry methods. iScience. (2021).
Figures