3429
Optimal control RF pulses for dual-angle T1 measurements in 31P magnetization transfer spectroscopy1Institute of Molecular Biosciences, University of Graz, Graz, Austria, 2Institute of Biomedical Imaging, Graz University of Technology, Graz, Austria, 3Institute for Mathematics and Scientific Computing, University of Graz, Graz, Austria
Synopsis
Keywords: Pulse Sequence Design, CEST & MT
Herein, we show for the first time the application of optimal control (OC) to the design of RF pulses for dual-angle T1 measurements as needed for 31P magnetization transfer spectroscopy. Phantom measurements highlight that OC RF pulses are able to produce precise T1 maps within the relevant coil's sensitivity range. The resulting enlarged spin ensemble leads to high SNR and excellent T1 prediction if used in non-selective spectroscopic application. In addition, OC pulses were optimized for operation at a large bandwidth suitable for 31P spectroscopy (± 15 ppm), which is often a problem when adiabatic BIR-4 pulses are used.
Introduction
The measurement of enzymatic exchange rates in vivo by magnetization transfer (MT) is a unique feature of magnetic resonance spectroscopy (MRS) [1,2]. 31P-MT spectroscopy relies on the accurate measurement of the apparent T1 of a phosphate group (e.g. inorganic phosphate (Pi)) which is under enzymatic exchange with a second phosphate group (e.g. γ - ATP through ATP synthase) upon saturation of the latter [3]. In a typical MT-MRS experiment, T1 needs to be validated at different binding-sites with distinct chemical shift offsets [4]. The low signal and therefore time consuming acquisition of phosphor MRS imposes two additional constraints: first, high SNR surface transmit/receive coils are used, and second, a fast two-angle T1 measurement is employed [5]. When using the two-angle method, the inhomogeneous transmitting field brings adiabatic excitation pulses into play [6,7]. However, robustness to frequency offsets and B+1 insensitivity of adiabatic excitation is limited [8]. Therefore, we propose the use of optimal control pulses [9,10] that provide high flip angle precision, strong B+1 insensitivity, and a bandwidth optimized for phosphor excitation at 7 Tesla.Methods
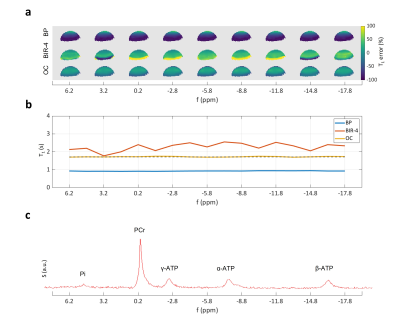
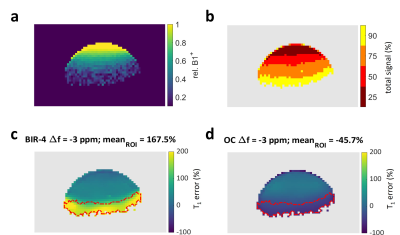
Measurements were carried out on a 7 Tesla platform (Bruker Biospec 70/20, Ettlingen, Germany) with a custom built 31P T/R surface coil of 11 mm inner diameter. In order to visualize coil (B+1) dependent errors in the T1 measurement, a 2D-slice phantom was built filling a 5 ml syringe with 475 μl of 98% ortho-phosphoric acid (4 mm slice). Dual-angle (α = 15° / β = 60°) T1 measurements were performed according to the work of Bottomley and Ouwerkerk [5]. The phosphoric acid singlet was fixed to -5.8 ppm (center of 31P spectrum with PCr assigned to 0 ppm). Stability to frequency offsets was tested by changing the center frequency of the excitation pulses from f = 6.2 ppm (Δf = 12 ppm) to f = -17.8 ppm (Δf = -12 ppm) in steps of -1.5 ppm. For excitation of the dual-angle T1 acquisitions, the following frequently used pulses were compared: hardpulse (BP, Tp = 0.05 ms, B+1 = 48.4/193.5 μT, 15°/60° resp.), adiabatic B1-independent rotation pulse based on a sech-tanh modulation function as designed in [8] including phase cycling [11] (BIR-4, ζ = 10, κ = atan(20), Δωmax = 3.5 kHz, Tp = 4 ms, peak B1+ = 500 μT), and a newly developed optimal control design pulse [10] optimized for T1 mapping in 31P-MT spectroscopy (OC, bw = ± 15 ppm, ΔB+1 = 0.4-1.2 x B+1nom, Tp = 1.64/2.47 ms, 15°/60° resp., peak B1 = 500 μT). The other imaging parameters were: TR = 800 ms, TE = 2 ms, FOV = 20 mm x 20 mm, matrix = 64 x 64, NEX = 12. A B+1 map was acquired with the same parameters as above, but with TR = 10 s, BP excitation and two flip angles α = 60° / β = 120° [12]. Images were masked corresponding to SNR > 0.1 · SNRmax of the OC(60°) scan. Ground truth T1 was determined as the mean of the voxels within ± 2.5 % B+1nom and the dual-angle measurement of the BP excitation.Results
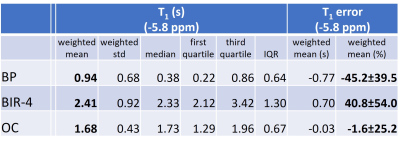
Figure 1 demonstrates the superior accuracy of OC excitation pulses optimized and applied to dual-angle T1 measurements in 31P-MT spectroscopy. Comparing OC with BIR-4 excitation, ΔB+1 and/or frequency offsets cause strong (yellow and dark blue coded) deviations of the BIR-4 measurement from the true T1 value (T1 = 1.71 ± 0.17 s). The signal distribution of the surface coil in Figure 2 (b,c) shows that those erroneous areas within the BIR-4 measurement account for approx. 20 % of the overall signal and thus significantly modulate the sum signal and hence the measured T1 (Fig. 1, b). T1 measurement based on BIR-4 excitation overestimates T1 throughout the whole bandwidth. This appears to be the result of incomplete phase compensation during the (phase cycled) composite parts of the pulse [11]. BP excitation underestimates T1 because lots of measured signal originate from spins that are under-excited, which directly leads to an underestimation of T1 [5]. OC design pulses performed best in measuring T1 till the outermost end of the coil detectable rim with the smallest mean error of -1.6 ± 25.2 % (mean ± standard deviation of the mean) compared to all RF pulses investigated (Figure 3).Discussion and Outlook
Based on phantom measurements we showed that OC outperform non-adiabatic (hardpulse) and adiabatic (BIR-4) excitation by accurate measuring T1 over the relevant coil's sensitivity range. Its successful application in MT spectroscopy depends on the consistent performance of the excitation over the observed chemical shift offsets. If T1 values depend (incorrectly) on the frequency offset of spins, as shown here for a phase corrected BIR-4 excitation, the recorded enzymatic exchange rates also exhibit - possibly critical - deviations depending on the binding site investigated. Therefore, for any quantitative analysis using MT spectroscopy, it is absolutely critical to choose the excitation that is most stable to variations in the desired Δ B+1 and chemical shift range, such as the OC excitation presented here. In this regard, the use of optimal control pulses is not limited to 31P and should be considered in any other application of X-nuclei spectroscopy.Acknowledgements
No acknowledgement found.References
[1] Befroy D. E. et al.: 31P-Magnetization Transfer Magnetic Resonance Spectroscopy Measurements of In Vivo Metabolism. Diabetes 2012;61(11):2669–2678 https://doi.org/10.2337/db12-0558
[2] Yuchi Liu, Yuning Gu, Xin Yu: Assessing tissue metabolism by phosphorous-31 magnetic resonance spectroscopy and imaging: a methodology review. QIMS, Vol 7, No 6, December 2017, https://doi.org/10.21037/qims.2017.11.03
[3] McConnell H.M.: Reaction Rates by Nuclear Magnetic Resonance. J. Chem. Phys. 28, 430 (1958); https://doi.org/10.1063/1.1744152
[4] Uǧurbil K.: Magnetization-transfer measurements of individual rate constants in the presence of multiple reactions. JMR Volume 64, Issue 2, September 1985, Pages 207-219 https://doi.org/10.1016/0022-2364(85)90345-2
[5] Bottomley P.A., Ouwerkerk R.: The Dual-Angle Method for Fast, Sensitive T1 Measurement in Vivo with Low-Angle Adiabatic Pulses. JMR, B, Volume 104, Issue 2, June 1994, Pages 159-167 https://doi.org/10.1006/jmrb.1994.1070
[6] De Graaf R.A., Nicolay K.: Adiabatic rf pulses. Applications to in vivo NMR. Concepts Magn Reson 9: 247–268, 1997 https://doi.org/10.1002/(SICI)1099-0534(1997)9:4<247::AID-CMR4>3.0.CO;2-Z
[7] Garwood M., Ke Y.: Symmetric pulses to induce arbitrary flip angles with compensation for rf inhomogeneity and resonance offsets. Journal of Magnetic Resonance (1969), Volume 94, Issue 3, p. 511-525. https://doi.org/10.1016/0022-2364(91)90137-I
[8] De Graaf R.A., Nicolay K.: Adiabatic rf pulses: Applications to in vivo NMR. Concepts in Magnetic Resonance (1997), Volume 9, Issue 4, p. 247-268. https://doi.org/10.1002/(SICI)1099-0534(1997)9:4<247::AID-CMR4>3.0.CO;2-Z
[9] Graf C. et al.: Advanced design of MRI inversion pulses for inhomogeneous field conditions by optimal control. NMR Biomed. 2022, https://doi.org/10.1002/nbm.4790
[10] Graf C. et al.: RF pulse optimization for robust excitation in 31P magnetization transfer spectroscopy. Submitted to ISMRM 2023.
[11] Bottomley P.A., Ouwerkerk R.: BIRP, an Improved Implementation of Low-Angle Adiabatic (BIR-4) Excitation Pulses. JMR. Volume 103, Issue 2, 15 June 1993, Pages 242-244 https://doi.org/10.1006/jmra.1993.1162
[12] Stollberger R., Wach P.: Imaging of the Active B1 Field inVivo. MRM. Volume 35, Issue 2, 1996, Pages 246-251 https://doi.org/10.1002/mrm.1910350217
Figures