3417
Isotropic sub-mm bSSFP imaging near metal at 0.55T
Kübra Keskin1, Nam G. Lee2, Jay Acharya3, and Krishna S. Nayak1,2
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 3Radiology, University of Southern California, Los Angeles, CA, United States
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 3Radiology, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Artifacts, Artifacts, Metal
At conventional field strengths, the large magnetic susceptibility difference between metallic implants and surrounding tissues causes severe artifacts often requiring multi-spectral imaging. Recent low-field MRI systems offers new opportunities for imaging adjacent to metallic implants, including balanced SSFP. Here, we utilize phase-cycled bSTAR, a short-TR 3D dual-echo radial bSSFP sequence, for isotropic 3D banding artifact free imaging near metallic implants at 0.55T. We demonstrate diagnostic quality images in volunteers with wrist and spinal hardware with high SNR efficiency compared to TSE-based sequences.Introduction
MRI near metallic implants is limited by the susceptibility difference between metallic implants and human tissues which causes field strength dependent field variations. This causes substantial artifacts and distortions around the implants in MR images1,2. Therefore, main field strength is a large factor affecting the size of metal artifacts3,4. At conventional field strengths, techniques like view angle tilting (VAT) and multi-spectral imaging (MSI) are used to reduce metal artifacts. Recent high-performance low field (<1.5T) systems allow opportunities for developing new techniques due to reduced field inhomogeneity. Here, we explore 3D radial balanced steady-state free precession (bSSFP) imaging to be used in imaging near metal.bSSFP is a fast-imaging sequence that provides unique contrast and high SNR, which is shown to be useful in many applications5, particularly for musculoskeletal6 and spine imaging7. However, its sensitivity to off-resonance causes signal voids in images, known as “banding” artifacts. Since the presence of metal perturbs the main magnetic field vastly in nearby regions, banding artifacts are prevalent around the metallic implants8. Banding artifact patterns can be shifted spatially by applying phase-cycling with different RF phase increments, and banding-free images can be obtained by combining multiple phase-cycled images9.
In this work, we explore bSTAR imaging10, a 3D bSSFP sequence based on dual-echo half-radial sampling with a short non-selective hard pulse excitation. With the relaxed SAR constraint at 0.55T, shorter duration RF pulses can be applied, which helps to excite the full spectral bandwidth of spins, while a non-selective RF pulse ensures no through plane distortions. Since shorter TR and TE values are possible with bSTAR, signal loss due to intra-voxel signal dephasing can be reduced. A high-resolution isotropic acquisition gives the freedom of reformatting images into an arbitrary plane. This enables 3D visualization of tissues near metallic implants, potentially eliminating another TSE-based scans at different orientations, and thus provides an opportunity to use bSSFP imaging near metallic implants at 0.55T.
Methods
All experiments were performed on a whole-body 0.55T scanner (prototype MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate). Data were collected using 6 elements of a table-integrated spine array (posterior) and a 6-channel body coil (anterior). 3D isotropic bSSFP imaging was performed with the bSTAR sequence, implemented with the Pulseq framework11. Image reconstruction was performed with BART12.A non-selective hard pulse with a duration of 180 μs was used for excitation. Imaging parameters were (phantom/in vivo): half-radial projections=20,000/40,000, 89 interleaves, FA=40°, readout bandwidth=1335/1116 Hz/Px, TR=1.82/2.12 ms, TE=0.12 ms, FOV=340x340x340 mm3, and isotropic resolution =1/0.89 mm3. Phase-cycling was applied with 8 different RF phase increments [0,π/4,π/2,3π/4,π,5π/4,3π/2,7π/4] for phantom and 4 RF phase increments [0,π/2,π,3π/2] for in vivo. Total scan times were 4 minutes 48 seconds and 5 minutes 40 seconds for phantom and in vivo experiments, respectively. Multi-acquisition phase-cycled bSSFP images were combined with sum-of-squares (SoS) to mitigate banding artifacts.
Performance of the sequences was tested with a phantom including spinal implant hardware placed into a water bath. One volunteer with a wrist plate and screws was imaged in the prone superman position. One volunteer with a vertebral body tethering system was imaged in the supine position. TSE with VAT and SEMAC images were also collected for comparison. Scan parameters are listed in Table 1. Volunteers were scanned under a protocol approved by our institutional review board after providing written informed consent.
Results
Figure 1 illustrates banding artifact patterns around spinal implant hardware created with 8 different phase-cycling RF increments. Sum-of-squares combinations of 4 and 8 phase-cycled bSSFP acquisitions are shown along with VAT and SEMAC.Figure 2 illustrates [0,π/2,π,3π/2] phase-cycled bSSFP images for volunteers with (a) wrist and (b) spinal hardware.
Figures 3 and 4 show banding-free bSSFP images after SoS combination in three orthogonal orientations, along with TSE with VAT, SEMAC images, and their reformats for wrist and spine, respectively. bSSFP provides sharp, banding-free reformats due to isotropic resolution and excellent contrast to evaluate the spinal canal. Structures in the spinal canal and around the hardware in axial bSSFP reformat can be seen better compared with TSE-based axial reformats and separate axial T2 acquisition.
Discussion
bSSFP creates reduced signal loss due to intra-voxel signal dephasing compared with gradient-echo based imaging due to its very short echo time. However, this signal loss is still present immediately adjacent to implant hardware, due to the lack of a spin-echo refocusing. This, in turn, creates a small signal void, which was noticeably larger than the void seen on TSE and SEMAC images; however, this was not deemed to alter clinical interpretation or confidence.Some metals (e.g., stainless steel) can cause very large field inhomogeneity13, causing spins to fall outside the frequency bandwidth of RF excitation. This could be mitigated by repeating bSSFP imaging with a shifted center frequency of RF excitation, similar to multi-spectral imaging1. Therefore, performance of bSSFP imaging near metal depends on implant material composition, main field strength, and imaging parameters.
Conclusion
We demonstrate isotropic sub-mm banding-free bSSFP imaging near metallic implants at 0.55T with phantom and in vivo scans. Tissue structures around the implants can be clearly seen compared with TSE-based acquisitions.Acknowledgements
We acknowledge grant support from the National Science Foundation (#1828736), research support from Siemens Healthineers, and USC Annenberg Graduate Fellowship (to K.K.). We thank Mary Yung for research coordination and Sophia X. Cui for collaboration.References
1. Koch KM, Hargreaves BA, Pauly KB, et al. Magnetic resonance imaging near metal implants. Journal of Magnetic Resonance Imaging 2010;32:773-787.2. Hargreaves B, Worters PW, Pauly KB, et al. Metal-induced artifacts in MRI. American Journal of Roentgenology, 2011;197(3):547-555.
3. Khodarahmi I, Brinkmann IM, Lin DJ, et al. New-Generation Low-Field Magnetic Resonance Imaging of Hip Arthroplasty Implants Using Slice Encoding for Metal Artifact Correction. Investigative Radiology, 2022;57(8):517-526.
4. Khodarahmi I, Keerthivasan MB, Brinkmann IM, et al. Modern Low-Field MRI of the Musculoskeletal System. Investigative Radiology, 2022;Publish Ahead of Print.
5. Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. European Radiology, 2003;13(11):2409-2418.
6. Gold GE, Hargreaves BA, Reeder SB, et al. Balanced SSFP imaging of the Musculoskeletal System. Journal of Magnetic Resonance Imaging, 2007;25(2):270-278.
7. Danagoulian GS, Qin L, Nayak KS, et al. Comparison of wideband steady-state free precession and T2-weighted fast spin echo in spine disorder assessment at 1.5 and 3 T. Magnetic Resonance in Medicine, 2012;68(5):1527-1535.
8. Hoff MN, Green JD, Xiang QS. Imaging Near Metals with Phase-Cycled SSFP. In Proceedings of the 18th Annual Meeting of ISMRM, 2010. P. 3081.
9. Bangerter NK, Hargreaves BA, Vasanawala SS, et al. Analysis of multiple-acquisition SSFP. Magnetic Resonance in Medicine, 2004;51(5):1038-1047.
10. Bieri O, Pusterla O, Bauman G. Free-breathing half-radial dual-echo balanced steady-state free precession thoracic imaging with wobbling Archimedean spiral pole trajectories. Zeitschrift Für Medizinische Physik, 2022.
11. Layton KJ, Kroboth S, Jia F, et al. Pulseq: A rapid and hardware-independent Pulse Sequence prototyping framework. Magnetic Resonance in Medicine, 2016;77(4):1544-1552.
12. Uecker M, Ong F, Tamir JI, et al. Berkeley Advanced Reconstruction Toolbox. In Proceedings of the 18th Annual Meeting of ISMRM, 2015. P. 2486.
13. Lee M, Kim S, Lee S, et al. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. RadioGraphics, 2007;27(3):791-803.
Figures
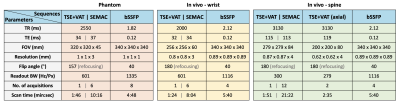
Table 1. Scan parameters (TR, TE, FOV, resolution, flip angle, readout bandwidth, number of acquisitions, and scan time) for phantom and in vivo experiments. Note that the number of acquisitions in TSE+VAT, SEMAC, and bSSFP correspond to the number of averages, SEMAC factor, and the number of phase-cycled acquisitions, respectively.
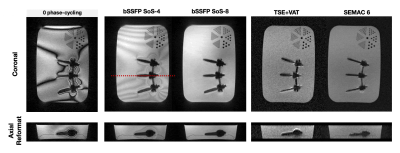
Figure 1. Illustration of banding artifact patterns around spinal hardware (8 different RF phase-cycling). SoS combinations of 4 and 8 phase-cycled bSSFP acquisitions are shown along with TSE+VAT and SEMAC 6. Red dashed line indicates the location of axial reformat. Note that bSSFP has a higher SNR efficiency, and no through-plane distortion as seen in TSE+VAT. The boundaries of screws are clear and easily distinguishable from its surroundings. Implants in bSSFP images appear slightly thicker compared with TSE-based sequences because of its irreversible intra-voxel dephasing.
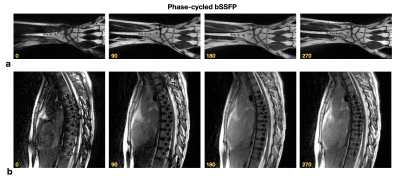
Figure 2. Illustration of 0, π/2, π, and, 3π/2 phase-cycled bSSFP images for volunteers with (a) wrist and (b) spinal hardware. Notice the alternation in banding artifact patterns with phase cycling, which helps to recover underlying signal while multiple phase-cycled images are combined.
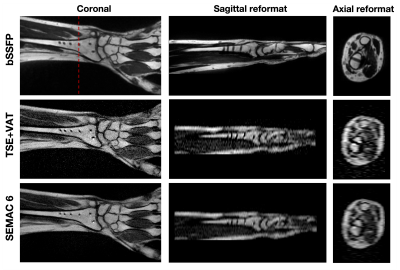
Figure 3. Banding-free bSSFP images after SoS combination in three orthogonal orientations for wrist, along with coronal TSE+VAT, SEMAC 6 images, and their sagittal and axial reformats. Red dashed line shows the location of axial reformat. Note that bSSFP provides sharp banding-free reformats due to its isotropic resolution. Although the size of signal voids around implants in bSSFP images is slightly larger compared with TSE+VAT and SEMAC 6 images as expected, any structure around implants is not obscured. Both TSE+VAT and SEMAC 6 images show small signal pile ups around implants.
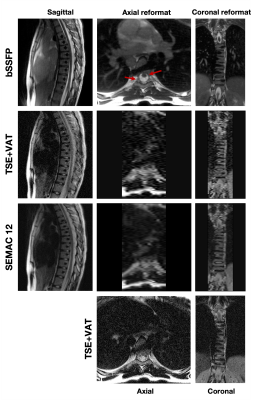
Figure 4. Banding-free bSSFP images after SoS combination in three orthogonal orientations for spine, along with sagittal TSE+VAT, SEMAC 12 images, their axial and coronal reformats, and axial TSE+VAT. Note that bSSFP provides sharp banding-free reformats due to its isotropic resolution and excellent contrast for spinal canal evaluation. Structures in the spinal canal around the spinal hardware in an axial bSSFP reformat (red arrows) can be clearly seen compared with TSE-based axial reformats and a separate axial T2 acquisition.
DOI: https://doi.org/10.58530/2023/3417