3406
Quantification of oxygenation levels within biocompatible hydrogels subcutaneously implanted in rats
Yuka Sugamura1, Vikram Kodibagkar1, Amy Emerson1, and Jessica Weaver1
1Arizona State University, Tempe, AZ, United States
1Arizona State University, Tempe, AZ, United States
Synopsis
Keywords: Quantitative Imaging, Oxygenation, pO2 mapping, T1 mapping, hydrogels
Cell therapy has the potential to repair irreversible pathologies including infarction and diabetes. High survival rate of the delivered cells is essential for effective treatment, where cell oxygenation is a critical factor. Biocompatible hydrogels are often used as cell delivery vehicles. Here, we propose an approach to quantify the oxygen availability that the cells encapsulated in biocompatible hydrogels experience after cell transplantation. In this study, we demonstrate the feasibility of assessing the pO2 within the hydrogels implanted in rats by combining the hydrogel with a previously developed magnetic resonance imaging (MRI) -based tissue oximetry technique.Introduction
Cell therapy has the potential to repair irreversible pathologies including infarction and diabetes. High survival rate of the delivered cells is essential for effective treatment, where cell oxygenation (pO2) is a critical factor. It is ideal that cell delivery vehicles are designed to have optimal oxygen retention properties. We have previously developed a magnetic resonance imaging (MRI)-based tissue oximetry technique called proton imaging of siloxanes to map tissue oxygenation levels (PISTOL). This utilizes siloxanes as MRI pO2 probes. The linear relationship between the spin-lattice relaxation rate (R1) of the siloxane protons and the surrounding pO2 allows quantitative and spatial pO2 mapping. We hypothesized that incorporating the pO2 probes into biocompatible hydrogels that can be used for cell delivery may help to assess the oxygen availability that the cells are experiencing after cell transplantation. Such information may aid improving the design of cell delivery vehicles by finding the optimal geometry and composition to maximize cell viability. Here we report the preliminary results of quantifying the pO2 within two types of hydrogels, agarose and alginate, implanted in rats.Materials and Method
Tetradecamethylhexasiloxane (L6) was used as MRI pO2 probes. Because L6 is highly hydrophobic, it was first combined with water, Solutol HS15, and Nile Red to result in an o/w emulsion. This emulsion was mixed with equal amounts of 4% agar aqueous solution, resulting in final concentrations of 20% L6 combined with 2% agarose or 1.5% alginate. Toxicity test was performed using INS-1E beta cells. Cells were encapsulated at 5 IEQ/µL in the hydrogels (20%L6/2%agarose or 20%L6/1.5%alginate) and the viability was evaluated at 48 hours using alamarBlue. In vitro experiments were carried out to determine the relationship between the R1 and pO2 for both hydrogel types. Hydrogels (disk shape, diameter and height were approximately 6 mm and 2 mm, respectively) were placed into a sample holder that allows maintaining the temperature at 37 ℃ and controlling the oxygen concentration. Samples were equilibrated under various oxygen concentrations (0, 5, 10, 15, and 21%O2) and scanned at 7T using the PISTOL sequence. The PISTOL sequence involves water and fat suppression, frequency-selective excitation of the siloxane protons and pulse-burst saturation recovery with an EPI readout for fast T1 measurement. TR was varied from 0.1 to 20 seconds. The acquired images were analyzed with a custom MATLAB code to generate a R1 map to yield the calibration curves. In the in vivo experiment, the L6/agar hydrogel and the control were subcutaneously implanted in the lower back of a rat. PISTOL scans were performed under two conditions, while the rats were breathing air and after switching the breathing gas to oxygen and having them breath for 20 minutes.Results and Discussion
Cell viability test using INS-1E beta cells showed that the incorporation of the L6 nanoemulsion into the hydrogels did not introduce any significant toxicity (Fig. 1). The relationship between the R1 and the pO2 under 37 C were determined to be R1 = 0.00108 * pO2 + 0.251 and R1 = 0.00082 * pO2 + 0.262 for L6/agarose and L6/alginate hydrogels, respectively (Fig. 2). Quantitative and spatial R1 maps were successfully obtained from both hydrogel types implanted in the lower back of the rats (n=3 for each type) (Fig. 3). The increase in local pO2 in response to switching the breathing gas from air to oxygen was captured. The pO2 values computed from one PISTOL scan appeared to have variability, especially under oxygen breathing conditions. This was expected, considering the potential inhomogeneity of oxygen distribution within the hydrogel which can arise from the variability in how the hydrogels have integrated with the surrounding tissue and their location. This range overlaps with the physiological pO2 range found in literature (40-120 torr)1.Conclusion
This study demonstrated the feasibility of quantifying the pO2 within two types of biocompatible hydrogels subcutaneously implanted in rats at locations where fat tissue is present in proximity. As a next step, we aim to perform pO2 mapping within a cell-laden system.Acknowledgements
This project is funded by NIH R01DK 129858-01. We are grateful to Samrat Amin for designing and fabricating the phantoms used for the calibration study.References
1. Wilson DF, Lee WM, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol (1985). 2006 Dec;101(6):1648-56. doi: 10.1152/japplphysiol.00394.2006. Epub 2006 Aug 3. PMID: 16888050.Figures
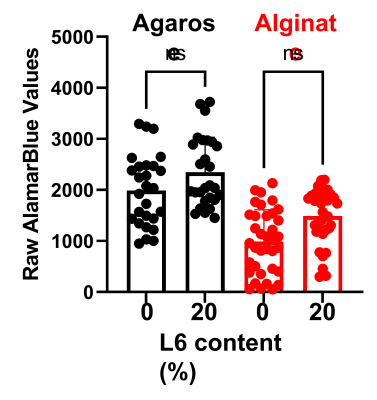
Cell viability within L6/agarose and L6/alginate hydrogels.
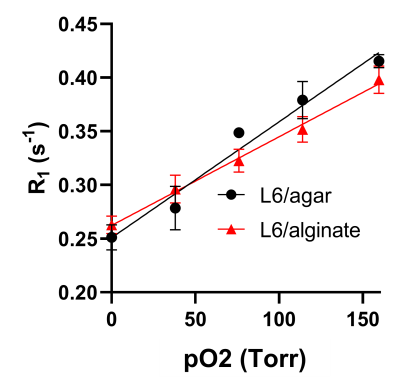
Calibration curves at 37 ˚C for L6/agarose and L6/alginate hydrogels.
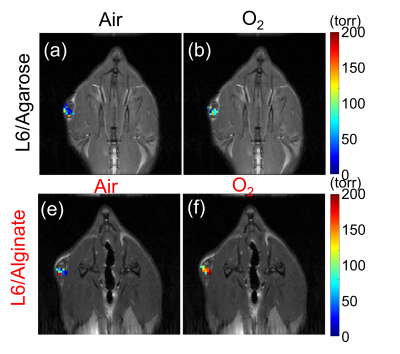
Representative pO2 maps under air and oxygen breathing with rats with L6/agarose (a,b) or L6/alginate (c,d) hydrogel implants.
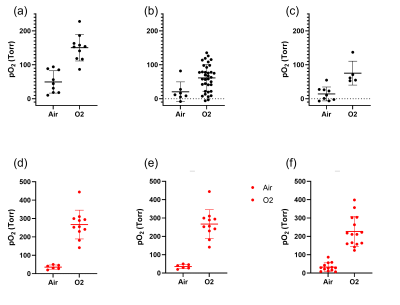
pO2 values measured from each pixel under air and oxygen breathing from the rats with L6/agarose (a-c) or L6/alginate (d-f) implants (n=3 for each).
DOI: https://doi.org/10.58530/2023/3406