3392
Comparison of a hybrid multi-delay pseudo-continuous arterial spin labelling scheme with time-encoded and variable-TR schemes1Philips Japan, Tokyo, Japan, 2Department of Molecular Imaging & Diagnosis, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 3C.J. Gorter MRI Center, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 4Division of Radiology, Department of Medical Technology, Kyushu University, Fukuoka, Japan, 5Department of Radiological Technology, Faculty of medical sciences, Kyoto College of Medical Science, Kyoto, Japan, 6Philips Healthcare, Best, Netherlands, 7Philips Healthcare, Tokyo, Japan
Synopsis
Keywords: Quantitative Imaging, Perfusion, ASL
To accurately calculate cerebral blood flow (CBF) and arterial transit time (ATT), there are two kinds of time-efficient approaches in use for multi-delay pseudo-continuous arterial spin labelling: time-encoded and sequential variable-TR. Hybrid schemes have recently been proposed that exploit the strengths of these approaches, but they have not been evaluated head-to-head, and the purpose of this study is therefore to investigate the clinical validity of hybrid schemes. The SNR, CBF and ATT were measured and compared in seven healthy subjects. A higher SNR was obtained in the hybrid scheme with high correlation coefficients with other ATT schemes, suggesting quantitative ability.Introduction
Arterial transit time (ATT) and cerebral blood flow (CBF) can be measured by multi-delay pseudo-continuous arterial spin labelling (pCASL), and two approaches have been proposed: time-encoded (TEnc) and sequential variable-TR (vTR).1–6 Due to its inherent noise-averaging effects, TEnc provides an signal to noise ratio (SNR) advantage when same label durations and post-label delays (PLD) are used,1 but because the label duration (LD) is split into blocks, there are stringent constraints on the possible combinations of LD and PLD. The vTR scheme allows flexible settings of LD and PLD without limitations,3,4,6 but SNR can only be increased by measuring more averages, which increase scan time significantly.Hybrids of both schemes (Hyb) have recently been proposed,7 which exploit the strengths and mitigate the weaknesses of TEnc and vTR, but such hybrids have not been evaluated head-to-head. This study therefore aims to investigate the quantitative ability of Hyb schemes in healthy volunteers’ examinations.
Methods
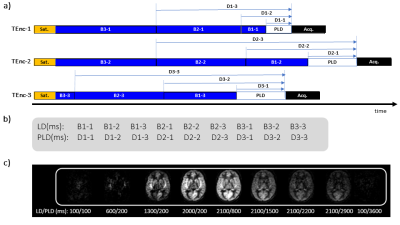
Hybrid pCASL SchemeFigure 1a shows an example nine-delay Hyb scheme comprising of three TEnc schemes with three LD blocks. TR is minimised, and the entire LD/PLD is rearranged and displayed in Figure 1b to show the effective LDs/PLDs. In the first phase, the LD is lengthened while the PLD is shortened and fixed, and in the second, the LD is fixed at the maximum and the PLD is lengthened, analogous to how contrast medium is administered. The final phase is assumed to be a super-delay phase in which the bolus will not provide any signal. Actual multi-delay images are displayed in Figure 1c.
Magnetic Resonance Experiments
The Hyb, vTR and TEnc schemes were implemented for comparison using a 3.0T Ingenia Elition scanner (Philips, Best, Netherlands). Two Hyb schemes were tested, one with 12 delays (Hyb12) and another with nine (Hyb9); 12 delays were used for the vTR scheme (vTR12) and seven for TEnc scheme (TEnc7). The actual LDs and PLDs used in this study are shown in Figure 2a. The equilibrium magnetization (M0) was acquired separately, and detailed sequence parameters are summarised in Figure 2b. Seven healthy subjects (mean age 39.3±10.7 years) were examined, and informed consent, as required by the Institutional Review Board, was obtained from all volunteers.
SNR, ATT and CBF Quantification
In the multi-delay series of each scheme, the three highest ASL signals along the time axis were selected and averaged voxel by voxel. The SD of the residual signal in grey matter (GM) was then calculated in the super-delay phase for the vTR and Hyb, and in the first delay phase for TEnc. The SNR was calculated by dividing the averaged ASL signal by the SD. CBF and ATT maps were created using a Buxton general kinetic model by applying a nonlinear fitting.5
Hybrid Scheme Validations
All SNR, CBF and ATT maps were normalised to the Montreal Neurological Institute space template. The averaged SNR and CBF in the GM region were calculated and compared between the schemes. Since the scan time differs for each scheme, time SNR (tSNR)—the SNR divided by the square root of the scan time—was used for comparison.
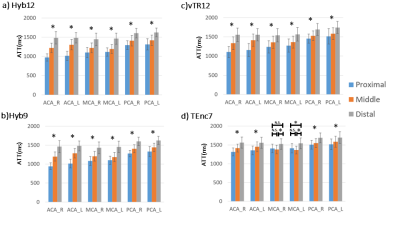
The volumes of interest were automatically delineated using a vascular territory atlas template,8 and the ATT values in the anterior cerebral artery (ACA), middle cerebral artery (MCA) and posterior cerebral artery (PCA) territories for both left and right hemisphere—with subdivision into proximal, middle and distal regions—were evaluated.
A P-value lower than 0.05 was considered statistical significant.
Results and Discussion
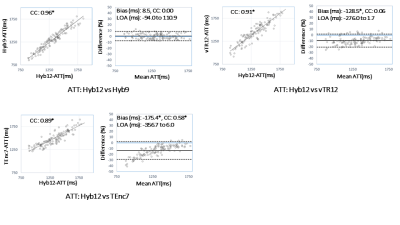
Representative multi-delay ASL images, and CBF and ATT maps for all schemes are shown in Figure 3, just as the tSNR and CBF in the GM. The tSNRs for Hyb9 and Hyb12 in the GM were significantly higher than those of vTR12 and TEnc7. The tSNR for Hyb9 was significantly higher than that of Hyb12, and significant differences in the CBF were observed between both vTR and TEnc and the two Hyb schemes, suggesting that scheme-dependent factors influenced the CBF measurement.ATT comparisons between the flow territories and regions are shown in Figure 4. For all flow territories, the ATT measured by Hyb and vTR schemes was significantly prolonged in the three regions in the order of proximal, middle and distal. Correlations and Bland-Altman plot analysis for ATT are shown in Figure 5. Statistically significant correlations were observed between Hyb12 and the other schemes. No significant systematic error was observed between Hyb12 and Hyb9. Significant fixed bias was observed between Hyb12 and vTR12, suggesting that scheme-dependent factors influenced the ATT measurement.
There were regions that showed no significant difference in ATT in the two flow territories in TEnc7 (Figure 4). In addition, significant fixed and proportional biases were observed between Hyb12 and TEnc7, suggesting less reliability for ATT measurement in TEnc7 because of limited LD/PLD combinations.
Conclusion
The quantitative validity of Hyb schemes is evidenced by the high SNR and proper ATT measurements. Since Hyb9 has higher tSNR and shorter scan time than Hyb12, with no significant differences in other comparisons, it is advantageous clinically. However, this study used healthy volunteers, and a clinical evaluation will be required in the future.Acknowledgements
No acknowledgement found.References
1. Dai, W., Shankaranarayanan, A. & Alsop, D. C. Volumetric measurement of perfusion and arterial transit delay using hadamard encoded continuous arterial spin labeling. Magn. Reson. Med. 69, 1014–1022 (2013).
2. Ishida, S. et al. Robust arterial transit time and cerebral blood flow estimation using combined acquisition of Hadamard-encoded multi-delay and long-labeled long-delay pseudo-continuous arterial spin labeling: a simulation and in vivo study. NMR Biomed. 33, 1–12 (2020).
3. Makoto Obara, Osamu Togao, Tatsuhiro Wada, Chiaki Tokunaga, Ryoji Mikayama, Hiroshi Hamano, Kim van de Ven, Masami Yoneyama, Tetsuo Ogino, Yuta Akamine, Yu Ueda, Jihun Kwon, Marc Van Cauteren. In Proc. Intl. Soc. Mag. Reson. Med. 29 870 (2021).
4. Osamu Togao, Makoto Obara, Kazufumi Kikuchi, Tatsuhiro Wada, Chiaki Tokunaga, Ryoji Mikayama, Shingo Baba, Marc Van Cauteren, Akio Hiwatashi, K. I. In Proc. Intl. Soc. Mag. Reson. Med. 30 4606 (2022).
5. Guo, J. et al. Comparing accuracy and reproducibility of sequential and Hadamard-encoded multi-delay PCASL for measuring CBF and ATT in healthy subjects – a simulation and in vivo study. J. Magn. Reson. Imaging 47, 1119–1132 (2018).
6. Johnston, M. E., Lu, K., Maldjian, J. A. & Jung, Y. Multi-TI arterial spin labeling MRI with variable TR and bolus duration for cerebral blood flow and arterial transit time mapping. IEEE Trans. Med. Imaging 34, 1392–1402 (2015).
7. Woods, J. G., Chappell, M. A. & Okell, T. W. Designing and comparing optimized pseudo-continuous Arterial Spin Labeling protocols for measurement of cerebral blood flow. Neuroimage 223, (2020).
8. Mutsaerts, H. J. M. M. et al. Cerebral perfusion measurements in elderly with hypertension using arterial spin labeling. PLoS One 10, 1–13 (2015).
Figures