3391
Current UK perspectives on the challenges for clinical translation of quantitative MR imaging biomarkers.1Centre for Medical Imaging, UCL, London, United Kingdom, 2Department of Neuroimaging,Institute of Psychiatry, King's College London, London, United Kingdom, 3Radiology Department, University College London, London, United Kingdom, 4Division of Cancer Sciences, The University of Manchester, Manchester, United Kingdom
Synopsis
Keywords: Quantitative Imaging, Challenges, Consensus
A web-based survey was developed from the “Steps on the Path to Clinical Translation” workshop, at the British & Irish Chapter-ISMRM conference held on 7th September 2022. The survey explored the UK MRI community’s perspective on the clinical translation of quantitative MR imaging biomarkers. Three main themes emerged from the results: the need for 1) consensus; continued development of resources that unite existing work and improve shared lexicon; 2) context dependency; defining the steps to clinical translation, in ways that appreciate the uniqueness of the imaging biomarker and the clinical question; 3) a clearer definition of imaging biomarker expectation or product profile.
Introduction
An interactive session at the British & Irish Chapter (BIC)-ISMRM Annual Meeting 2022 Clinical Translation Workshop “Steps on the Path to Clinical Translation” asked attendees to address 7 areas pertinent to improving clinical translation for quantitative MR (qMR) imaging biomarkers (IBs). The conclusions/ further questions that resulted from discussions were developed into a survey. We aimed to investigate the UK-based MRI community’s perspectives on major obstacles in clinically translating qMR IBs, and what actions could be useful to address them.Methods
A REDCap project, was used to derive a web-based e-survey and a QR/quick access link distributed as an open invitation. The survey, open for 5 weeks, was made available during the 2022 BIC-ISMRM Annual Meeting and subsequently distributed via communication emails/newsletters to the BIC-ISMRM, MR-PHYSICS, British Society of Neuroradiologists (BSNR) and various institutional emailing lists. The survey was made of 40 questions covering 7 topics (Fig. 1). Descriptive statistics were drawn from multiple choice (MCQ), Likert and agree/disagree questions, and a thematic analysis with a deductive approach1 was performed on free text questions to identify themes across topics. Basic occupational demographic questions allowed us to monitor MRI community representation.Results
We received 101 responses with completion varying from 27.7–100%. Responses were received from imaging scientists (research (42.6%) and clinical (11.9%)), clinicians (6.9%), others (5%) and 33.7% chose not to disclose.Three significant themes emerged from thematic analysis across all free text questions.
· Consensus
The need to build consensus and resources dedicated to improving the standardisation of certain aspects of IB development: (i) terminology, (ii) decision making and (iii) validation (Q 1B, 1Bii, 3B, 3D, 4E, 5C, and 5D). Views were frequently expressed on: (i) the format for sharing consensus, (ii) what consensus building should target, (iii) the lack of multi-disciplinary guidelines, (iv) standardisation of terminology, (v) the need for action groups and (vi) for publications on how to define the pathway. Questions 1Bi, 2B, 3A and 3C show that respondents agree with the need to improve consensus (Fig. 2)
· Context dependency
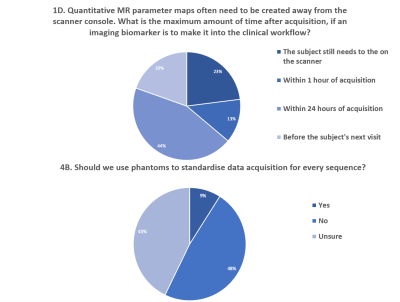
The theme of context dependency was frequently raised across all topics: (i) phantoms, (ii) terminology, (iii) decision making and (iv) end-points (Q 1Ai, 1D, 2Ai, 2Bi, 4Bi and 7B). Views were expressed strongly regarding the uniqueness of each clinical situation when integrating IBs into the clinical workflow. The nature of this integration raised variability in views, but most frequently, that the context should be developed based on the IBs themselves, closely followed by the clinical question, availability, and cost. Questions 4B and 1D had high uncertainty, further expression in follow-up questions, 4Bi and 4E, stated the need for context dependency (Fig.3).
· Expectations of the IB (Product Profile)
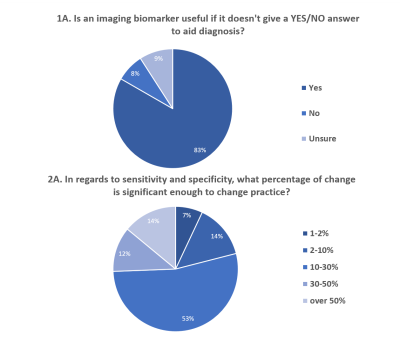
When considering the product profile2 (Q1Ai, 1D, 2Ai, 2Bi, 2Ci and 7A), opinions strongly favoured qMR IBs supplementing, rather than replacing existing pathways. Many considered that a specific end-point of an IB in clinical practice would have a staged approach, although this varied when considering IBs across patient groups or along a treatment process. For example, in question 2Ci, many stated that the ideal situation would be to target clinical translation of an IB for a single situation/patient group initially and then, following subsequent phased research/ development, transfer the IB to other patient pathways. Respondents often stated conversations required increased input from a larger breadth of stakeholders and networks, such as vendors and national/international societies involved in qMR. A clear product profile can aid the research design and end translation of a medical test3, but more input from the MRI community would be required to establish clear product profiles for individual qMR IBs. Questions 1A and 2A looked at the product profile and had a more even distribution of responses and follow-up remarks than for other survey questions (Fig. 4).
Conclusion
Our survey demonstrates that, according to a national cohort of the MRI community in the UK, there remain unmet needs and challenges in improving the pathway to clinical translation of qMRI IBs. Published consensus often addresses the context dependency by a siloed approach, but our results suggest that a more standardised, but adaptive approach, would allow a common ground that could have larger benefit. A move to a more standardised consensus was observed in recommendations for Arterial Spin Labelling (ASL), where a more structured and holistic approach was developed in published work4 than in published work5. Recent consensus building has developed guidance for a range of IBs in broader contexts6,7, nevertheless consensus methodology is highly variable and more work is needed to establish the more standardised, but adaptive, approach that was suggested by the survey results.We plan to build on this study by providing a forum for continued action and “widening the conversation” by gaining an international perspective during the ISMRM 2023 conference. We hope to develop a multifaceted and successful approach to addressing the many, and diverse, needs and challenges in translating qMR IBs into the clinic.
Acknowledgements
We would like to thank the British & Irish Chapter ISMRM organisers for hosting the workshop “Steps on the Path to Clinical Translation” and for their help in distributing the online survey.
Our independent research is supported by the National Cancer Imaging Translation Accelerator (NCITA). Views expressed represent the views of the authors and not necessarily those of NCITA.
References
1. Michelle E. Kiger & Lara Varpio (2020) Thematic analysis of qualitative data: AMEE Guide No. 131, Medical Teacher, 42:8, 846-854, DOI: 10.1080/0142159X.2020.1755030
2. Cocco P, Ayaz-Shah A, Messenger MP, West RM, Shinkins B. Target Product Profiles for medical tests: a systematic review of current methods. BMC Med. 2020 May 11;18(1):119. doi: 10.1186/s12916-020-01582-1.
3. Terry RF, Plasència A, Reeder JC. Analysis of the Health Product Profile Directory - a new tool to inform priority-setting in global public health. Health Res Policy Syst. 2019 Dec 12;17(1):97. doi: 10.1186/s12961-019-0507-1.
4. Nery F, Buchanan CE, Harteveld AA, Odudu A, Bane O, Cox EF, et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. Magn Reson Mater Physics, Biol Med 2020;33:141–61. https://doi.org/10.1007/s10334-019-00800-z
5. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102–16. https://doi.org/10.1002/mrm.25197
6. deSouza, N.M., Achten, E., Alberich-Bayarri, A. et al. Validated imaging biomarkers as decision-making tools in clinical trials and routine practice: current status and recommendations from the EIBALL* subcommittee of the European Society of Radiology (ESR). Insights Imaging 10, 87 (2019). https://doi.org/10.1186/s13244-019-0764-0
7. O'Connor JP, Aboagye EO, Adams JE, Aerts HJ. et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017 Mar;14(3):169-186. doi: 10.1038/nrclinonc.2016.162.
Figures
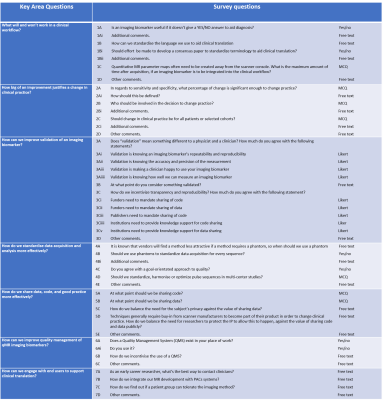
Figure 1. Survey composition: Seven key areas related to the clinical translation of quantitative MR imaging biomarkers were developed into a follow-on survey at the interactive BIC-ISMRM workshop, “Steps on the Path to Clinical Translation”. Here we outline the survey questions and detail the question type.
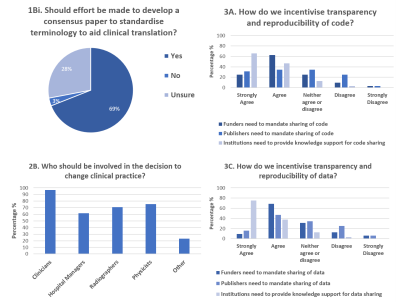
Figure 2. Consensus building: Question 1Bi demonstrated 69% of respondents wanted consensus papers to standardise terminology. Question 2B demonstrated that it was expected that translating an imaging biomarker would require a breadth of input from the MRI community, with 87.7% of respondents indicating that 2 or more groups should be involved in decision making. Question 3A and 3C showed that more respondents strongly agreed/agreed that institutions needed to provide knowledge support, than felt funder or publishers should mandate sharing.