3390
Altered amide proton transfer weighted signal and diffusion in multiple sclerosis: correlation with neuroflament light and disease duration1Xuanwu Hospital, Capital Medical University, Beijing, China, 2Philips Healthcare, Beijing, China
Synopsis
Keywords: Multiple Sclerosis, CEST & MT
APTw imaging can help us understand the pathological changes in MS more sensitively and accurately.Introduction
Multiple sclerosis (MS) is a central nervous system (CNS) inflammatory demyelinating disease that mostly affects young and middle-aged people and is associated with a high prevalence of neurological impairment. MS has an alternating relapse-remitting clinical history in its early stages, with relapses frequently defined by acute periods of neurological impairments, depending on the location of the lesion and the degree of the inflammatory process. Amide proton transfer weighted (APTw) imaging is a molecular MRI approach that does not require the injection of any contrast agent and imaging of the content and enviorment of amide protons in tissue endogenous mobile cell proteins and peptides. Diffusion tensor imaging (DTI) assesses white matter (WM) fiber tract damage in an indirect manner and reveals microscopic abnormalities. The aim of this study was to evaluate and compare the efficacy of APTw and ADC, FA in evaluating white matter lesions in MS patients, as well as to see whether lesions' APTw values were associated with clinical features such as serum neuroflament light (sNfL).Materials and methods
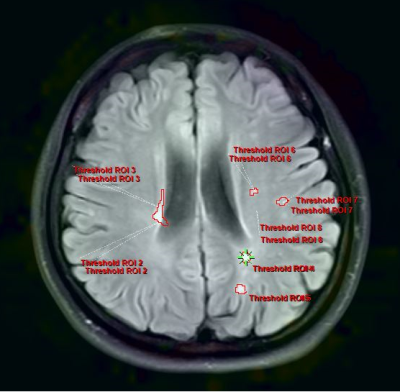
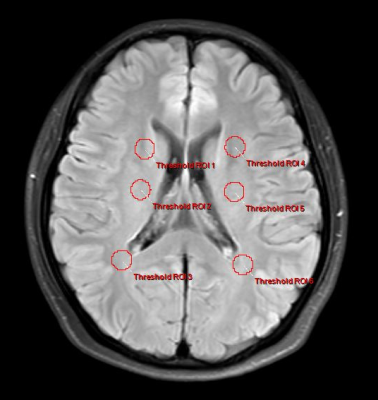
29 individuals with relapsing-remitting MS (21 females and 8 males) were recruited. Patients with a relapsing-remitting course who met the 2017 McDonald criteria were eligible. None of these patients had relapsed or been treated with drugs (e.g., interferon-beta or immunosuppressive therapy) during the three months preceding the MRI. Meanwhile, 30 healthy controls (HCs; 23 females and 7 males) with no history of neurologic impairment and normal results on neurologic examination were included as the control group. The MRI was performed using a 3.0-T MR system (Ingenia, Philips Healthcare, Best, the Netherlands) with an 8-channel head coil. APTw data were obtained using a 3D-Dixon TSE imaging sequence with the following parameters: repetition time (TR) / echo time (TE) = 5874 / 7.8 ms; flip angle = 90°; slice thickness = 6 mm; gap = 0 mm; in-plane resolution, 2 mm 2 mm; FOV 230 mm × 182 mm; matrix size = 116 × 91 DWI sequence (TR/TE = 1600/2.13 ms, inversion time (TI) = 1000 ms, flip angle = 9°, FOV = 256 mm × 224 mm, matrix size = 256 × 224, slice thickness = 1.0 mm, voxel dimensions = 1.0 mm × 1.0 mm). APTw pictures were registered to FLAIR-SPIR images on the postprocessing workstation of "IntelliSpace Portal" (version 9, Philips Healthcare, The Netherlands) and assessed by two neuroradiologists. The ROI criteria were: 1) On axial FLAIR-SPIR images of MS patients, each lesion was identified (Fig. 1). APTw values for MS are calculated using mean values from all lesions. 2) According to the previous reference, the white matter around the lateral ventricle (frontal lobe, parietal lobe, and centrum semiovale) of each participant was assessed bilaterally (Fig. 2).Results
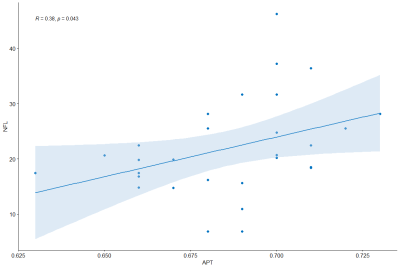
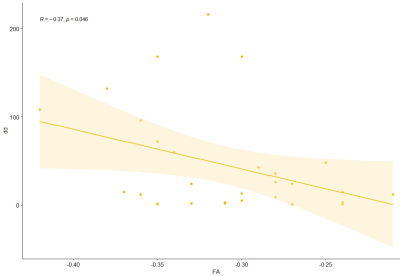
A two-sample t-test analysis revealed that APTw values of brain lesions in MS patients were significantly higher than in HCs (P = 0.00). Furthermore, the ADC values of MS patients were significantly higher than those of HCs (P = 0.00). FA levels were significantly lower than HCs (P = 0.00). sNfL was considerably positively connected with APTw (P = 0.043, R = 0.38) (Fig. 3), but disease durations were significantly negatively correlated with FA (P = 0.046, R = -0.37) (Fig. 4).DISCUSSION and CONCLUSIONS
The current study evaluated the cellular and molecular imaging evaluations of APTw and DTI for brain lesions in MS patients. According to our preliminary findings, these two MRI approaches give extra quantitative clinical diagnostic information. APTw imaging can help us understand the pathological changes in MS more sensitively and accurately, and APTw value has a high association with clinical factors. These associations between APT parameters and clinical factors imply that APT may play a role in monitoring disease impairment.Acknowledgements
The authors would like to thank the participants for their commitment.References
1. Marisa P McGinley, Carolyn H Goldschmidt, Alexander D Rae-Grant; Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA.2021;325(8):765-779.
2. Cristina Granziera, Jens Wuerfel, Frederik Barkhof, et al; Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain : a journal of neurology 2021;144(5):1296-1311.
3. Jinyuan Zhou, Hye-Young Heo, Linda Knutsson, et al; APT-weighted MRI: Techniques, current neuro applications, and challenging issues. Journal of magnetic resonance imaging : JMRI 2019 ;50(2):347-364.
4. Hye-Young Heo, Zheng Han, Shanshan Jiang,et al; Quantifying amide proton exchange rate and concentration in chemical exchange saturation transfer imaging of the human brain. NeuroImage 2019;189:202-213.
5. Tao Liu, Yanrong Chen, Aline M Thomas, et al; CEST MRI with distribution-based analysis for assessment of early stage disease activity in a mouse model of multiple sclerosis: An initial study. NMR in biomedicine 2019;32(11):e4139.
6. Adrienne N Dula, Elizabeth M Asche, Bennett A Landman, et al; Development of chemical exchange saturation transfer at 7 T. Magnetic resonance in medicine 2011;66(3):831-8.
7. Pascal Benkert, Stephanie Meier, Sabine Schaedelin, et al; Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. The Lancet. Neurology 2022;21(3):246-257.