3387
Improved Characterization of White Matter in Multiple Sclerosis with Free Water-Corrected Diffusion MRI1Bristol Myers Squibb, Lawrenceville, NJ, United States, 2Imeka Solutions, Inc., Sherbrooke, QC, Canada, 3Biogen, Cambridge, MA, United States, 4Cytel, Waltham, MA, United States
Synopsis
Keywords: Multiple Sclerosis, Diffusion/other diffusion imaging techniques
Free water (FW)-corrected metrics in diffusion MRI have shown promise in elucidating confounding pathological processes in multiple sclerosis (MS). Here, we present a retrospective analysis of FW-corrected diffusion in a cohort of 287 MS participants from a multi-center clinical trial. Our preliminary results indicate the method’s ability to distinguish different lesion types and MS subtypes.Introduction
Diffusion MRI (dMRI) is a mainstay in many neurological clinical assessments and clinical trials. Free water (FW) in dMRI can be measured when the conventional diffusion tensor model is expanded to include an isotropic component to fit the unconstrained water signal within a voxel1. In studies of multiple sclerosis (MS) patients, elevated FW content has been associated with neuroinflammation2,3, while elevated radial diffusivity (RD) has been demonstrated to be a strong predictor of demyelination and axonal loss4. Here, we present a retrospective analysis of FW-corrected dMRI, pooling data from a large phase 2 clinical trial.Methods
Baseline data was pooled from the SYNERGY clinical trial (NCT01864148): 287 multiple sclerosis participants (RRMS n=232, SPMS n=55) and 49 healthy volunteers from 70 different sites. The imaging protocol consisted of high resolution T1-W (1.2 mm isotropic or 1.2 x 0.9 x 0.9 mm3), pre- and post- contrast-enhanced T1-W (0.97 x 0.97 x 3 mm3), T2-W and diffusion (in-plane resolution=1.25x1.25 mm2 or 2.5x2.5 mm2, slice thickness=2.5-3 mm, number of unique directions= 13-31, b-value=1000 s/mm2, averages=1-2) imaging. Imaging was performed on both 1.5T and 3T systems.All diffusion datasets were pre-processed for artifact removal (denoising, motion correction, and eddy current correction)5. Diffusion maps were generated based on 1) the conventional diffusion tensor imaging (DTI) model and 2) a bi-tensor model incorporating free water content estimation.
Thirty-three bundles were extracted and analyzed. Regions of interests along specific bundles were delineated on the T2-W and pre- and post-contrast T1-W images, with the following MS lesion categories: acute (identified as gadolinium-enhancing from the post-contrast T1-W images), chronic black hole CBH (identified as hypointense from the pre-contrast T1-W images), pre-existing T2 (identified as hyperintense from the T2-W images), and normal appearing white matter NAWM (identified by removing all lesions from the whole tract). For healthy volunteer comparisons, the whole tract was assessed. Nonparametric Wilcoxon rank sum tests were performed to determine whether statistical differences between field strengths, lesion types and MS subtypes were observed for the derived metrics.
Results
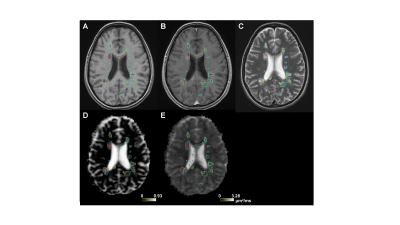
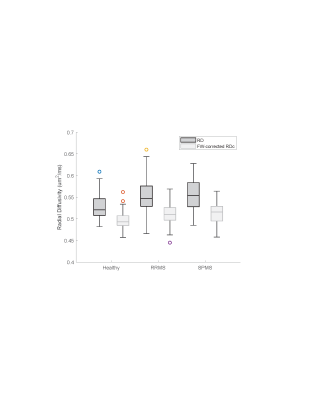
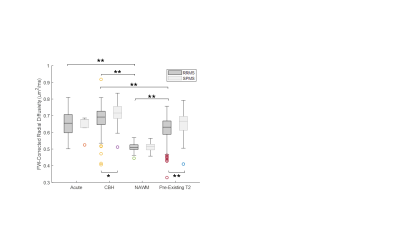
For brevity, only FW and RD metrics in the corticospinal tract (CST) are presented. FW-corrected DTI was successfully implemented in this multi-center context. Notably, in healthy controls across sites, DTI-derived and FW-corrected diffusion metrics were consistent across field strengths (RD: p=0.13, FW: p=0.06, FW-corrected RDc: p=0.27). Figure 1 shows a representative MS participant with conventional imaging (Fig. 1a, 1b, 1c), along with the FW map (Fig. 1d) and FW-corrected RDc map (Fig. 1e). As shown in Figure 2, when FW correction was applied, the DTI-derived metric RDc yielded lower values and resulted in lower variability in the whole tract of healthy subjects (RD: 0.52±0.03, RDc: 0.50±0.02 µm2/ms), the NAWM of RRMS participants (RD: 0.55±0.03, RDc: 0.51±0.02 µm2/ms) and the NAWM of SPMS participants (RD: 0.56±0.04, RDc: 0.51±0.02 µm2/ms), though the contribution of FW content in healthy controls was smaller than in the NAWM of MS participants, as expected (healthy: 3.1±1.4%, RRMS: 4.1±1.6%, SPMS: 4.4±1.7%). Lastly, Figure 3 demonstrates the potential of FW-corrected diffusion for further distinguishing lesion types as well as MS subtypes. In RRMS participants, FW-corrected RDc can differentiate NAWM from acute, CBH and pre-existing T2 lesions (p<0.0001) and CBH lesions from pre-existing T2 lesions (p<0.0001). FW-corrected RDc may also highlight differences in CBH lesions (p<0.01) and pre-existing T2 lesions (p<0.0001) between RRMS and SPMS participants.Discussion
The results herein are focused based on the interest of biomarkers sensitive for neuroprotective therapies (RD) and edema (FW content); the results within CST are presented due to its clinical impact on motor function. To our knowledge, we present the implementation of dMRI with FW correction in the largest multi-site, multi-field, and multi-vendor cohort of MS patients to date. Our data suggests the potential of FW-corrected RDc in distinguishing lesion types, potentially between MS subtypes, which may aid in characterizing the response of lesions in MS clinical trials. The current data was limited due to the varying diffusion sequence across sites and incorporation of only one b-value shell. Additionally, healthy controls were not age or sex matched. Future work will include longitudinal assessment of the derived metrics to determine the method’s ability in elucidating lesion evolution. Furthermore, correlation of metrics to clinical outcomes will be studied to help confirm the clinical utility of the findings.Acknowledgements
No acknowledgement found.References
1. Pasternak O, Sochen N, Gur Y, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009: 62(3): 717-30.
2. Kim M, Choi KS, Hyun RC, et al. Free-water diffusion tensor imaging detects occult periependymal abnormality in the AQP4-IgG-seropositive neuromyelitis optica spectrum disorder. Sci Rep. 2022:12(512).
3. Beaudoin A, Rheault F, Theaud G, et al. Modern Technology in Multi-Shell Diffusion MRI Reveals Diffuse White Matter Changes in Young Adults With Relapsing-Remitting Multiple Sclerosis. Front Neurosci. 2021:15.
4. Schmierer K, Wheeler-Kingshott CA, Tozer DJ, et al. Quantitative magnetic resonance of post-mortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59(2):268–277.
5. Theaud G, Houde JC, Boré A, et al. TractoFlow: A robust, efficient, and reproducible diffusion MRI pipeline leveraging Nextflow & Singularity. NeuroImage. 2020;218.
Figures