3377
Cingulum network myelination structure by SHIFT MRI characterizes higher order cognitive function in multiple sclerosis
Tatiana Wolfe1, Ashley Pike1, Sienna Colonese2, Carlos Ernesto Garrido Salmon3, Laura B. Dunn1, R. Lee Archer4, Clint D. Kilts1, and G. Andrew James1
1Psychiatry, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 2Neurosciences, Colorado State University, Denver, CO, United States, 3Physics, University of Sao Paulo, Ribeirao Preto, Brazil, 4Neurology, University of Arkansas for Medical Sciences, Little Rock, AR, United States
1Psychiatry, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 2Neurosciences, Colorado State University, Denver, CO, United States, 3Physics, University of Sao Paulo, Ribeirao Preto, Brazil, 4Neurology, University of Arkansas for Medical Sciences, Little Rock, AR, United States
Synopsis
Keywords: Multiple Sclerosis, Brain Connectivity, Myelination integrity, cognitive impairment
Impairment of higher-order cognition is a difficult aspect of MS pathology that poses a long-standing challenge for patient care advancement. Understanding how myelination integrity in the cingulum network – a brain pathway implicated in cognition – is related to lost or preserved cognitive functions is fundamental to enable comprehensive MS care and personalized remyelination therapeutics. We investigated if myelination integrity data has the potential to aid in characterizing individual differences in information processing in MS patients. Our findings support that myelination structure-function measured by SHIFT MRI is elucidatory variable to describe individual differences in higher-order cognitive function in MS patients.Introduction
The diversity of retained neurological functions among people living with multiple sclerosis (MS) is perhaps one of the most intriguing facets of human demyelinating pathologies.1 Demyelination lesions measured by magnetic resonance image (MRI) are the clinical hallmark for MS diagnosis and evolution, and are associated with varying patterns of neurologic dysfunction both epidemiologically and in the context of a single patient’s progression.2, 3 In relapsing-remitting MS, in particular, this exacerbated individual variability in neurofunctional presentation that is a correlated to the varying nature of lesion distributions across the brain poses a long-lasting difficult challenge for advancement of patient care.4, 5 Although MS is most prominently associated with motor deficits, MS lesion burden is increasingly associated with cognitive deficits even in the absence of motor impairment.3, 6-7 Specifically, demyelinating MS lesions found in the cingulum bundle and its network throughout the cerebral lobes, and along its associations to the corticothalamic fibers, have been linked to a panorama of critical cognitive dysfunctions, including disability in information processing, memory, social interaction and emotional control.8-10 Understanding how myelination integrity in the cingulum network relates to the preservation or loss of higher-order cognitive functions among MS patients could help facilitate more personalized, comprehensive clinical care, including effective remyelination therapeutics. We hypothesize that SHIFT MRI measurement of myelination integrity along the cingulum network is an elucidatory variable mediating individual differences in information processing function in patients with MS. Here, we preliminarily investigate this hypothesis by comparing myelination integrity patterns in two MS patients selected based on nearly identical total lesion volumes but differing information processing speed abilities.Methods
Medical, behavioral, anatomical, and functional MRI data was collected from two female patients (age 40.2 and 40.9 years) living with relapse-remitting MS who are class-matched in other secondary variables. Anatomical images (MPRAGE and T2-FLAIR) were acquired at 1 mm isotropic resolution, followed by a myelin SHIFT MRI and a diffusion spectrum imaging scan with 96-directions and b-values = 665, 1335, 1995 s/mm2. The symbols-digit modality test (SDMT)11 was administered during a consequent 6 minutes functional MRI scan with TR = 800 ms, and all data coded in BIDS.12 Images of all contrasts were co-registered using FSL and warped into MNI using statistical parametric mapping nonlinear registration.13 Freesurfer ‘samseg’ function was used to segment apparent lesions.14 Myelin integrity maps were computed by SHIFT MRI as previously described.15, 16 Using DSI studio, the q-space diffeomorphic reconstruction method was used to compute the quantitative anisotropy (QA) map in each subject’s native space. A deterministic tracking algorithm with angular cutoff of 35o, 1 mm step-size, 5/300 mm min./max. length, null spin-density smoothing, iterative QA threshold of 0.4 to the cumulated tract, and 25 topology pruning iterations was employed to delineate 2 million fibers along the cingulum network, generating a DSI graph for each dataset.17 The individual graph of the cingulum network was then used to extract myelination integrity histograms from the respective SHIFT MRI maps, and mean, mode, variance and kurtosis were computed. Standard processing pipelines were adopted for functional MRI analysis using fmriprep, and independent components were extracted in the preprocessing step to allow network-level analyses of cost switch on task-related neural function computed in the subject’s native space.Results
Information processing speed ability differed dramatically between subjects (SDMT_high-performer = 70, and SDMT_low-performer = 37) despite the nearly identical normalized total lesion volumes, respectively equal to 1.49% and 1.30% (Fig.1). The network degrees (hubness) computed in DSI (Fig.2) were found to differ between subjects (left cingulum = 18.2/12.0; the inferior longitudinal fasciculus = 21/32). The corresponding myelin SHIFT histograms computed in the left cingulum network were characterized by SHIFT T2 mean = 43.8/45.6 ms, mode = 57.0/101.0 ms, variance = 1466.8/879.2 ms, and kurtosis = 1.74/0.67, which demonstrates a trend of longer myelin SHIFT T2s (e.g. degeneration) is present along the left cingulum network in the lower but not in the higher performer (Fig.3). A flipped pattern was observed in the contralateral cingulum network (right): mean = 135.3/49.6 ms, mode = 167.1/385.9 ms, variance >2000 ms for both, and kurtosis = 0.27/2.26, respectively. The task functional MRI maps (Fig.4) show precuneus and left caudal anterior-cingulate activation (symbols>numbers) in the high-performer, while the lower-performer presents sparse activation in precuneus and prefrontal cortex.Discussion
Myelination integrity maps by SHIFT MRI provide a fine characterization of the structure-function aspects of the cingulum network, while quantitatively expressing aspects of the network’s connectome and related neuroprocessing abilities. The kurtosis of the myelin SHIFT T2 histogram computed for the involved network, which is a tangible quantity related to features of tract myelination integrity, appears to be a valid indicator of whether neurofunction is preserved or compromised throughout the implicated network. Future work involving larger samples is necessary to determine robustness and reliability of this new strategy. Furthermore, this preliminary study demonstrates the information of myelination integrity harnessed by SHIFT MRI – the longer myelin SHIFT T2s that originate from early onsetting neurodegenerations (e.g. enlargement of the periaxonal space of myelinated neurons) – has the potential to enrich multimodal imaging models of anatomy-function potentially improving predictive power at the individual level, and thereby improving targeted care to patients living with MS.Acknowledgements
The authors acknowledge the strong support of the UAMS Brain Imaging Research Center and the Rampy MS Research Foundation, and are thankful for the insightful support of the Writing Group in the UAMS Psychiatric Research Institute. This work is jointly supported by the Depart. of Psychiatry, by the Depart. of Neurology and the TRI at UAMS.References
- Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006 Jun 15;245(1-2):41-6. doi: 10.1016/j.jns.2005.08.019. Epub 2006 Apr 27. PMID: 16643953.
- Rocca MA, Sormani MP, Rovaris M, Caputo D, Ghezzi A, Montanari E, Bertolotto A, Laroni A, Bergamaschi R, Martinelli V, Comi G, Filippi M. Long-term disability progression in primary progressive multiple sclerosis: a 15-year study. Brain. 2017 Nov 1;140(11):2814-2819. doi: 10.1093/brain/awx250. PMID: 29053836.
- Hulst HE, Steenwijk MD, Versteeg A, Pouwels PJ, Vrenken H, Uitdehaag BM, Polman CH, Geurts JJ, Barkhof F. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013 Mar 12;80(11):1025-32. doi: 10.1212/WNL.0b013e31828726cc. Epub 2013 Mar 6. PMID: 23468546.
- Enzinger C, DeLuca J. Large-scale neuronal network dysfunction in multiple sclerosis?: Evidence from resting-state fMRI. Neurology. 2012 Oct 2;79(14):1416-7. doi: 10.1212/WNL.0b013e31826d600d. Epub 2012 Sep 5. PMID: 22955132.
- Andravizou A, Siokas V, Artemiadis A, Bakirtzis C, Aloizou AM, Grigoriadis N, Kosmidis MH, Nasios G, Messinis L, Hadjigeorgiou G, Dardiotis E, Peristeri E. Clinically reliable cognitive decline in relapsing remitting multiple sclerosis: Is it the tip of the iceberg? Neurol Res. 2020 Jul;42(7):575-586. doi: 10.1080/01616412.2020.1761175. Epub 2020 May 19. PMID: 32427076.
- Andravizou A, Siokas V, Artemiadis A, Bakirtzis C, Aloizou AM, Grigoriadis N, Kosmidis MH, Nasios G, Messinis L, Hadjigeorgiou G, Dardiotis E, Peristeri E. Clinically reliable cognitive decline in relapsing remitting multiple sclerosis: Is it the tip of the iceberg? Neurol Res. 2020 Jul;42(7):575-586. doi: 10.1080/01616412.2020.1761175. Epub 2020 May 19. PMID: 32427076.
- Abel S, Vavasour I, Lee LE, Johnson P, Ackermans N, Chan J, Dvorak A, Schabas A, Wiggermann V, Tam R, Kuan AJ, Morrow SA, Wilken J, Laule C, Rauscher A, Bhan V, Sayao AL, Devonshire V, Li DK, Carruthers R, Traboulsee A, Kolind SH. Myelin Damage in Normal Appearing White Matter Contributes to Impaired Cognitive Processing Speed in Multiple Sclerosis. J Neuroimaging. 2020 Mar;30(2):205-211. doi: 10.1111/jon.12679. Epub 2019 Nov 24. PMID: 31762132.
- Keser Z, Hasan KM, Mwangi B, Gabr RE, Steinberg JL, Wilken J, Wolinsky JS, Nelson FM. Limbic Pathway Correlates of Cognitive Impairment in Multiple Sclerosis. J Neuroimaging. 2017 Jan;27(1):37-42. doi: 10.1111/jon.12381. Epub 2016 Aug 19. PMID: 27541485; PMCID: PMC5226860.
- Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A, Bove M, Brichetto G, Uccelli A, Mancardi G, Roccatagliata L. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler. 2015 Apr;21(4):442-7. doi: 10.1177/1352458514546791. Epub 2014 Aug 21. PMID: 25145692.
- Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M. Impaired functional integration in multiple sclerosis: a graph theory study. Brain Struct Funct. 2016 Jan;221(1):115-31. doi: 10.1007/s00429-014-0896-4. Epub 2014 Sep 26. PMID: 25257603.
- Bensa, C. et al. [Early detection of cognitive impairment in relapsing-remitting multiple sclerosis: functional-anatomical correlations and longitudinal follow-up]. Rev. Neurol. (Paris) 162, 1221–1231 (2006).
- Brain Image Data Structure (BIDS). 2019-2022. https://bids.neuroimaging.io
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-19. doi: 10.1016/j.neuroimage.2004.07.051. PMID: 15501092.
- Cerri S, Puonti O, Meier DS, Wuerfel J, Mühlau M, Siebner HR, Van Leemput K. A contrast-adaptive method for simultaneous whole-brain and lesion segmentation in multiple sclerosis. Neuroimage. 2021 Jan 15;225:117471. doi: 10.1016/j.neuroimage.2020.117471. Epub 2020 Oct 22. PMID: 33099007; PMCID: PMC7856304.
- Wolfe T, Hoffman K, Hogan MK, Salazar B, Tang X, Chaboub L, Quini CC, Lu ZL, Horner PJ. Quantification of Myelinated Nerve Fraction and Degeneration in Spinal Cord Neuropil by SHIFT MRI. J Magn Reson Imaging. United States; 2021 Apr;53(4):1162–1174. PMID: 33098256
- Wolfe T, Quini C, Horner PJ, Hogan MK, Signal isolation magnetic resonance image (simri) and methods thereof, WO2020060997A1 (https://patentimages.storage.googleapis.com/28/67/9b/4d17af884e4a16/WO2020060997A1.pdf).
- Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WY. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013 Nov 15;8(11):e80713. doi: 10.1371/journal.pone.0080713. PMID: 24348913; PMCID: PMC3858183.
Figures
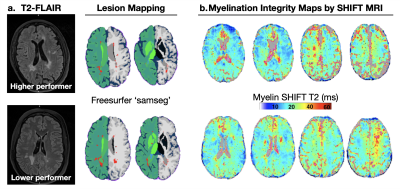
Fig. 1 - Myelin related views of the brain of MS patients (1.a) who scored higher in the symbol digit modality task (SDMT) of information processing (top row; SDMT_score = 70), and of a class-matched one who scored lower (bottom; SDMT_score = 37). The total relative lesion volume were 1.49%/1.30%, respectively. Myelination integrity maps by SHIFT MRI reveal differences in the longer myelin SHIFT T2 patterns across the white matter. Longer SHIFT T2s have direct association to periaxonal enlargement in myelinated neurons — a characteristic of early/mild onsetting neurodegenerations.
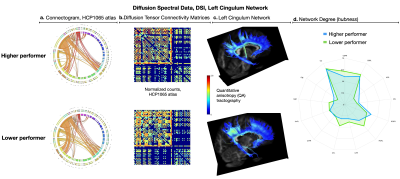
Fig. 2 - Left cingulum network properties — a tract implicated in cognition — shown for the higher and lower (top/bottom) task performers. Abbreviations in the connectograms (2.a), matrices (2.b), and spider-plot (2.d) follow the HCP1065 atlas coding. Connectome differences include lesions' disposition visible in the connectivity matrices (i.e. greater sparsity in the lower performer’s data), and in the QA tractography (2.c) where denser tracts (brighter yellow-green QA range) were detected along both the dorsal anterior and the prefrontal portions of the left cingulum network.
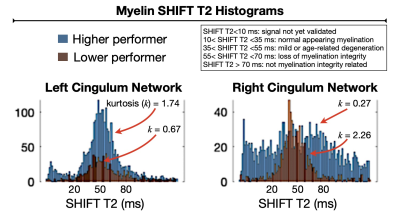
Fig. 3 - Myelination integrity measurements by SHIFT MRI elucidate tangible aspects of the fine structure of the cingulum association networks. It considers data from demyelinating lesions and includes information on concomitant mild neurodegenerative changes detected along the tracts (e.g. myelin SHIFT T2 between 35-55 ms). The kurtosis (k) computed for each SHIFT T2 histogram appears to be an indicator of the likelihood of preserved or compromised structure-function along the implicated pathway.
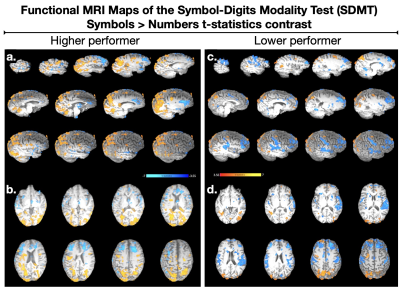
Fig. 4 - Neuro activation patterns measured by functional MRI (fMRI) during performance of the SDMT task. The striking functional differences between higher (4.a-b) and lower (4.c-d) performers reveal individualized brain representations of the information processing regions, which must occur atop of the implicated white matter networks mapped in Figs. 1-3. The delicate differences in myelination integrity measured by SHIFT MRI appear to elucidate the structure-function characteristics of individual differences in neurofunction among these MS patients.
DOI: https://doi.org/10.58530/2023/3377