3371
White matter macrostructure simultaneously predicts perceived rejection and cognitive inflexibility in late life
Tatiana Wolfe1, Carlos Ernesto Garrido Salmon2, G. Andrew James1, Laura B. Dunn1, and Clint Kilts1
1Psychiatry, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 2Physics, University of Sao Paulo, Ribeirao Preto, Brazil
1Psychiatry, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 2Physics, University of Sao Paulo, Ribeirao Preto, Brazil
Synopsis
Keywords: White Matter, Neuroscience, Neurodegeneration, Cognitive Flexibility, Late Life, Psychiatric Disorders' Risk Factors.
The interplay between brain white matter health and cognitive flexibility in late life is intimate. Understanding the age-related patterns of white matter disintegration along the cingulum network will aid in elucidating factors underlying individual susceptibility to psychiatric illness related cognitive flexibility impairment. We evaluated Bayesian Pearson correlations between a measurement of T1/T2-FLAIR kurtosis and psychiatric risk factors available in a UK Biobank sample. Our findings strongly suggest (BF10>100) that MRI apparent integrity loss in the cingulum, uncinate fascicles and corticothalamic fibers is a significant corollary of an older-age exclusive effect of increased perceived rejection that co-occurs with cognitive flexibility decline.Introduction
Cognitive flexibility is defined as the ability to update and shift executive functions in response to changing environmental demands.1 Cognitive flexibility is fundamental to humans’ ability to form and maintain social connection, yet it declines in late life.2-4 Recent evidence has implicated cognitive flexibility as a potential risk factor for psychiatric disorders in older adults.5, 6 Although white matter integrity has been found to be intimately associated with cognitive flexibility,7, 8 a comprehensive understanding of factors underlying vulnerability to impaired cognitive flexibility in late life remains lacking. Moreover, brain white matter thinning and atrophy are known effects of social disconnection.4, The cingulum bundle, a pathway connecting all cerebral lobes, is prominently involved in granting functional awareness to environmental stimuli, and its network is implicated in the dynamic regulation of cognitive flexibility.9 Recently, the specialized circuitry roles of the cingulum have been associated with both higher order functions in healthy controls, as well as with limbic dysfunctions in psychiatric disorders (e.g. bipolar disorder, schizophrenia, autism).10-11 Understanding the age-related patterns of white matter degradation along the cingulum interconnecting network will help elucidate key neurobiological factors contributing to impairment of cognitive flexibility impairment that may lead to psychiatric illness in late life. We theorize that white matter integrity along the cingulum and its network measured by MRI apparent texture is a significant explanatory variable of individual differences in cognitive flexibility impairments that occur in older adults who endorse higher levels of perceived social isolation. Here, we test for associations between white matter integrity and the age-related social disconnection-induced cognitive flexibility impairments.Methods
Using a curated sample of the UK Biobank dataset (n=121 older ages≥60 years and 180 younger adults ages 18-42 years), we evaluated the age-dependent relationships between white matter macrostructural integrity in the cingulum network, established sensitive measurements of cognitive flexibility performance (NIH toolbox Flanker Inhibitory and Dimensional Change Cart Sorting task), and aspects of social emotion (perceived friendship/loneliness) and functioning (perceived hostility/rejection), which are established markers of social disconnection.12 Student’s t-test was employed to evaluate group age-relate differences in the studied functional abilities. Anatomical images of each subject were co-registered, normalized, skull-stripped, and resampled to 1 mm isotropic resolution using FSL, and templated to MNI coordinates (Fig. 1.a).13 White matter segmentations of 66 cerebral and subcortical regions were performed in subject’s native space using the HCP1065 atlas in FSL (Fig. 1.b). From each anatomical region, histogram distributions of T1-pixel intensity and T1/T2-FLAIR were computed in Matlab. White matter macrostructural integrity was evaluated in terms of the T1 mean±variance (i.e. MRI apparent texture; m±v in Fig. 1c) and the kurtosis of the T1/T2-FLAIR ratio (k in Fig. 1.d) calculated for ipsilateral associations of the cingulum, arcuate and uncinate fasciculi, the commissural corpus callosum crossings, and the corticothalamic projections. To estimate correlation coefficients while considering the uncertainty of the estimated m, v and k, Bayesian Person correlations were computed in JASP, and significance is reported at Bayes factor BF10>100.Results
Inhibition and dimensional switch abilities displayed significant age-related effects (Fig. 2.a; p<0.001). Friendship scores wereinversely correlated to perceived rejection in older adults (Fig. 2.b right; r = -0.467), and self-reported loneliness was correlated to both perceived rejection and hostility in late life (Fig. 2.b left; r = 0.616, and r = 0.342 [graph not shown], respectively), evidencing that aspects of social emotion were strongly associated to social functioning in older adults exclusively. Inhibition and dimensional switch abilities were observed to correlate in both age groups (Fig. 2.c; r = 0.511, Fig. 2.d r = 0.583 in older and younger adults, respectively, with BF10>100). Remarkably, the analysis showed very strong evidence (BF10>100) of correlation between MRI apparent texture, m±v, and the inhibition domain of cognitive flexibility (r < -0.47 for all considered white matter structures). Individual differences in k, which report MRI-apparent textural variance in each white matter structure while also considering the textural influence of lesions visible in T2-FLAIR exams (i.e. hyperintensities), were also significantly correlated with perceived rejection in older adults exclusively (rUncinate_Left = 0.447, rCorthicothalamic_Right = 0.286, rCorthicothalamic_Left = 0.347).Discussion
Our findings suggest that apparent loss of integrity in the cingulum, and along its associative circuit with the uncinate fascicles and the corticothalamic fibers is a significant corollary of an older-age exclusive effect of increased perceived rejection that co-occurs with decline in cognitive flexibility. These findings provide novel evidence for a late-life specific relationship between the integrity of association tracts that concomitantly regulate cognitive flexibility and aspects of healthy social functioning. Our findings are limited by the macroscopic effects of white matter thinning that are visible in 3T MRI (i.e. measurements of apparent texture).14 Refined measurements of cingulum myelination integrity and function are imperative to further understand the mechanisms underlying late-life vulnerability to critical decline in cognitive flexibility.15, 16 Fine measurements of white matter integrity in these brain components have the potential to serve both as individualized markers of vulnerability to psychiatric conditions, and as targets for disease-modifying therapies. Future studies may include refined analyses and measurements of network structure and function, and further examine the possible mediating links between anatomical factors of age-related susceptibility to critical social disconnection-induced cognitive flexibility impairments.Acknowledgements
The authors acknowledge the strong support of the UAMS Brain Imaging Research Center, and are thankful for the insightful support of the Writing Group in the UAMS Psychiatric Research Institute.References
- Uddin LQ. Cognitive and behavioral flexibility: neural mechanisms and clinical considerations. Nat Rev Neurosci. 2021 Mar;22(3):167–179. PMCID: PMC7856857
- Laland K, Seed A. Understanding Human Cognitive Uniqueness. Annu Rev Psychol. United States; 2021 Jan 4;72:689–716. PMID: 33400565
- Kupis L, Goodman ZT, Kornfeld S, Hoang S, Romero C, Dirks B, Dehoney J, Chang C, Spreng RN, Nomi JS, Uddin LQ. Brain Dynamics Underlying Cognitive Flexibility Across the Lifespan. Cereb Cortex. 2021 Oct 1;31(11):5263–5274. PMCID: PMC8491685
- Zhang J, Liu D, Fu P, Liu ZQ, Lai C, Yang CQ, Chen K, Bao WD, Hu F, Du HY, Yang W, Wang J, Man HY, Lu Y, Zhu LQ. Social isolation reinforces aging-related behavioral inflexibility by promoting neuronal necroptosis in basolateral amygdala. Mol Psychiatry. 2022 Jul 15;1–14. PMCID: PMC9284973
- Park J, Moghaddam B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience. 2017 Mar 14;345:193–202. PMCID: PMC5159328
- Charak R, Byllesby BM, Fowler JC, Sharp C, Elhai JD, Frueh BC. Assessment of the revised Difficulties in Emotion Regulation Scales among adolescents and adults with severe mental illness. Psychiatry Res. Ireland; 2019 Sep;279:278–283. PMID: 30975439
- Phillips KA, Watson CM, Bearman A, Knippenberg AR, Adams J, Ross C, Tardif SD. Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. Am J Primatol. 2019 Feb;81(2):e22949. PMCID: PMC6685070
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011 Feb;134(Pt 2):449–463. PMCID: PMC3030764
- Leber AB, Turk-Browne NB, Chun MM. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008 Sep 9;105(36):13592–13597. PMCID: PMC2527350
- Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018 Sep;92:104-127. doi: 10.1016/j.neubiorev.2018.05.008. Epub 2018 May 16. PMID: 29753752; PMCID: PMC6090091
- Koshiyama D, Fukunaga M, Okada N, Morita K, Nemoto K, Usui K, Yamamori H, Yasuda Y, Fujimoto M, Kudo N, Azechi H, Watanabe Y, Hashimoto N, Narita H, Kusumi I, Ohi K, Shimada T, Kataoka Y, Yamamoto M, Ozaki N, Okada G, Okamoto Y, Harada K, Matsuo K, Yamasue H, Abe O, Hashimoto R, Takahashi T, Hori T, Nakataki M, Onitsuka T, Holleran L, Jahanshad N, van Erp TGM, Turner J, Donohoe G, Thompson PM, Kasai K, Hashimoto R; COCORO. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol Psychiatry. 2020 Apr;25(4):883-895. doi: 10.1038/s41380-019-0553-7. Epub 2019 Nov 29. PMID: 31780770; PMCID: PMC7156346
- Spreng RN, Setton R, Alter U, et al. Neurocognitive aging data release with behavioral, structural and multi-echo functional MRI measures. Scientific Data. 2022 Mar;9(1):119. DOI: 10.1038/s41597-022-01231-7. PMID: 35351925; PMCID: PMC8964687
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-19. doi: 10.1016/j.neuroimage.2004.07.051. PMID: 15501092.
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, Perry ML, Parvizi J, Hua le H, Butts-Pauly K, Wandell BA. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med. 2013 Dec;19(12):1667-72. doi: 10.1038/nm.3390. Epub 2013 Nov 3. PMID: 24185694; PMCID: PMC3855886.
- Wolfe T, Hoffman K, Hogan MK, Salazar B, Tang X, Chaboub L, Quini CC, Lu ZL, Horner PJ. Quantification of Myelinated Nerve Fraction and Degeneration in Spinal Cord Neuropil by SHIFT MRI. J Magn Reson Imaging. United States; 2021 Apr;53(4):1162–1174. PMID: 33098256
- Medaglia JD, Huang W, Karuza EA, Kelkar A, Thompson-Schill SL, Ribeiro A, Bassett DS. Functional Alignment with Anatomical Networks is Associated with Cognitive Flexibility. Nat Hum Behav. 2018;2(2):156–164. PMCID: PMC6258039
Figures
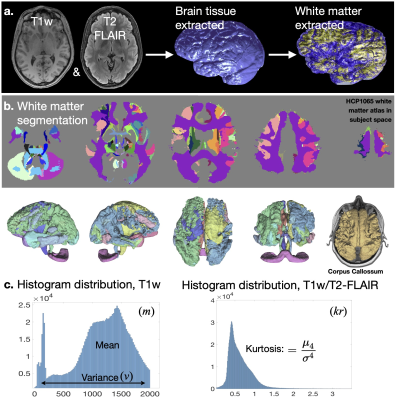
Fig. 1 – Measurements of apparent white matter integrity extracted from 3T MRI. Following registration (1.a) and white matter segmentation in FLS (1.b), the mean (m) and variance (v) of T1-weighted pixel intensity (1.c left) and the kurtosis (k) of the T1/T2-FLAIR histograms (1.c right) are computed for each atlas-segmented structure. The cingulum bundles, arcuate and uncinate fasciculi, corpus callossum associations, and the corticothalamic projections were considered in the analyses.
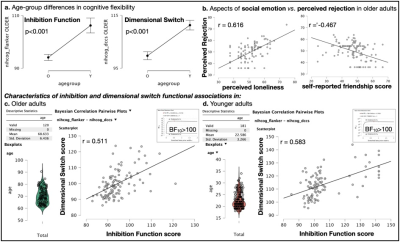
Fig. 2 – Cognitive flexibility and social functioning displayed significant age-related effects (2.a) for which older adults carry significant vulnerability to perceiving rejection (2.b). These correlations were not found to be significant among younger adults (not shown). Inhibition ability was significantly associated with dimensional switching function in both age groups (2.c-2.d).
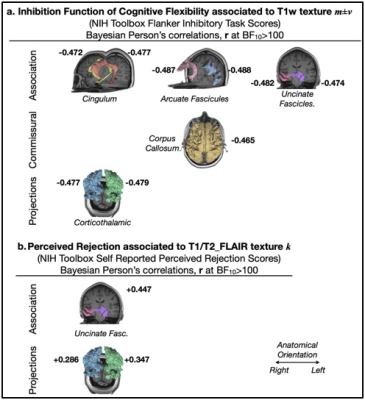
Fig. 3 – Properties of 3T MRI apparent texture, T1w mean and variance, m±v, and the kurtosis, k, of T1/T2-FLAIR histograms computed per segmented white matter bundle showed very strong evidence (BF10>100) of correlations with aspects of both cognitive flexibility (inhibition ability) and social functioning (perceived rejection), elucidating the possible mediating role of white matter integrity in late-life specific vulnerability to co-occurring perceived rejection and cognitive flexibility impairment.
DOI: https://doi.org/10.58530/2023/3371