3363
Abnormal cerebral micro-structures in end-stage renal disease patients related to mild cognitive impairment1Department of Radiology, The Affiliated Changzhou NO.2 People’s Hospital of Nanjing Medical University, changzhou, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
Keywords: Gray Matter, Diffusion/other diffusion imaging techniques
This study aimed to investigate the abnormal cerebral micro-structures related to mild cognitive impairment (MCI) and further predict individual cognitive function in end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis. Specially, diffusion kurtosis imaging (DKI), mediation analysis, and the least squares support vector regression machine (LSSVRM) were utilized to conduct our study. We observed that aberrant micro-structures partially mediated the association between clinical risk factors and MCI, which is a novel insight into the progression of cognitive dysfunction. The combination of DKI metrics and clinical characteristics could be used as features to efficiently predict cognitive function associated with ESRD.Introduction
This study aimed to investigate the abnormal cerebral micro-structures related to mild cognitive impairment (MCI) and further predict individual cognitive function in end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis. Specially, diffusion kurtosis imaging (DKI), mediation analysis, and the least squares support vector regression machine (LSSVRM) were utilized to conduct our study. We observed that aberrant micro-structures partially mediated the association between clinical risk factors and MCI, which is a novel insight into the progression of cognitive dysfunction. The combination of DKI metrics and clinical characteristics could be used as features to efficiently predict cognitive function associated with ESRD.Materials and Methods
Subjects: In total, 40 ESRD patients and 30 healthy controls were prospectively enrolled in our study. Both groups were matched based on age, gender, and education years. All subjects were right-handed and capable of independently completing the Montreal cognitive assessment scale (MoCA).MR protocols: MRI data were acquired with a 3.0T magnetic resonance scanner (Discovery MR750; Milwaukee, WI, USA) using a standard 32-channel head and spine combined coil. High-resolution anatomic T1-weighted images were acquired with three-dimensional brain volume imaging (3D-BRAVO) sequence (152 slices; slice thickness = 1.2 mm (no gap); TR = 8.2 ms; TE = 3.2 ms; FA = 12°; matrix = 256×256; FOV = 240 mm×240 mm; whole scanning time =3 min 57 s). DKI data were acquired with a single-shot echo-planar imaging (SS-EPI) sequence (30 directions; 3 b values: 0, 1000, 2000 s/mm2, NEX = 2, slice thickness = 3.6 mm (no gap); TR=6500 ms; TE = 95.8 ms; matrix = 128×128; FOV= 240 mm×240 mm; whole scanning time =14 min 43 s).
Data analysis: DKI data were processed with FMRIB Software Library (FSL) software and Diffusion Kurtosis Estimator (DKE). The quadratic programming-based (CLLS-QP) algorithm embedded in DKE was applied to calculate kurtosis parameters, including mean kurtosis (MK), axial kurtosis (AK), radial kurtosis (RK), and kurtosis anisotropy (KA).
A voxel-based two-sample t-test was used to detect differences in DKI parameters between the ESRD and healthy control groups based on GRETNA. Pearson correlation analyses were performed to investigate the relationships between DKI metrics, clinical characteristics, and cognitive scores among ESRD patients. Mediation analyses were performed to determine where the micro-structural alterations could mediate the role of clinical indicators in MCI. Clinical blood biochemistry indicators constituted the independent variable, MoCA score was the dependent variable and the altered DKI metrics constituted the mediator variable. Finally, optimized LSSVRM was used to predict cognitive scores based on DKI metrics and clinical risk factors.
Results
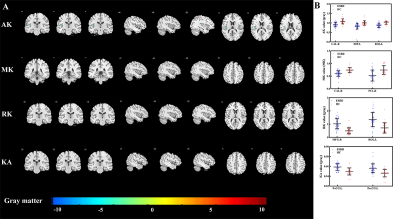
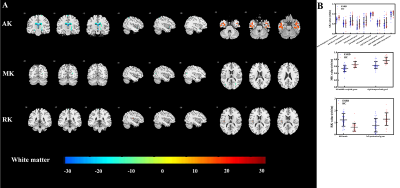
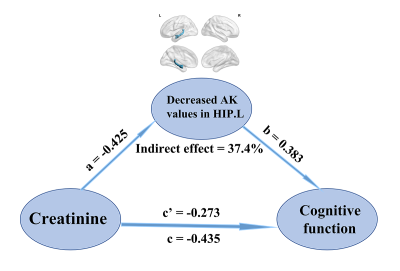
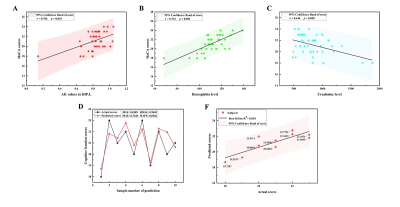
The voxel-based analysis detected 9 GM regions and 13 WM regions with significantly changed DKI metrics in ESRD patients (Fig 1 and Fig 2). The WM abnormalities were more widespread than GM abnormalities. From the GM analysis, decreased AK and MK were found in five regions, while increased RK and KA were found in four regions. From the WM analysis, decreased AK, MK, and RK were mainly found in the frontal and parietal lobes, increased AK and RK were mainly found in the temporal lobe, and no significant KA alteration was found. In the ESRD group, significant correlations were found among DKI metrics in GM, clinical characteristics, and cognitive scores. Mediation analysis indicated that decreased AK in the left hippocampus partially mediated the effects of serum creatine level on the cognitive function deficits (indirect effect = 37.4%) in ESRD patients (Fig 3). Optimized LSSVRM based on combined DKI metrics and clinical characteristics (decreased AK in the left hippocampus, hemoglobin level, and serum creatine level) predicted the cognitive function of ESRD patients with great accuracy. The mean square error (MSE), root mean square error (RMSE), mean absolute error (MAE), mean absolute percentage error (MAPE), and R-squared (R2) values between the actual scores and predicted scores were 0.72, 0.85, 0.75, 3.57%, and 0.55, respectively (Fig 4).Discussion and Conclusions
This study evaluated alterations of micro-structures related to MCI by using DKI in ESRD patients. DKI analysis showed that both the GM and WM presented disrupted integrity in ESRD patients with MCI. Mediation analysis demonstrated that the association between serum creatine level and MCI was partially mediated by decreased AK in the left hippocampus in ESRD patients. According to the LSSVRM model, the AK value of the left hippocampus, hemoglobin level, and serum creatine level could be effective features to objectively predict individual cognitive function with relatively high accuracy in ESRD patients.This study highlighted the important role of fused DKI metrics and blood biochemical indexes to predict cognitive function. And this may indicate the feasibility of earlier and more effective diagnosis of MCI associated with ESRD.
Acknowledgements
No acknowledgement found.References
1. Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. LANCET.10302 (398) (2021) 786-802. 10.1016/S0140-6736(21)00519-5
2. Vega JN, Newhouse PA. Mild Cognitive Impairment: Diagnosis, Longitudinal Course, and Emerging Treatments. CURR PSYCHIAT REP.10 (16) (2014) 10.1007/s11920-014-0490-8
3. Li A, Mu J, Huang M, Zhang Z, Liu J, Zhang M. Altered amygdala-related structural covariance and resting-state functional connectivity in end-stage renal disease patients. METAB BRAIN DIS.5 (33) (2018) 1471-1481. 10.1007/s11011-018-0254-y
4. Stenberg J, Eikenes L, Moen KG, Vik A, Håberg AK, Skandsen T. Acute Diffusion Tensor and Kurtosis Imaging and Outcome following Mild Traumatic Brain Injury. J NEUROTRAUM.18 (38) (2021) 2560-2571. 10.1089/neu.2021.0074
Figures